-
PDF
- Split View
-
Views
-
Cite
Cite
Lei Yang, Xinfang Liu, Yeyang Wang, Surgical treatment of ankylosing spondylitis with Andersson lesion in the thoracic spine: a case report and review of the literature, Journal of Surgical Case Reports, Volume 2025, Issue 6, June 2025, rjaf436, https://doi.org/10.1093/jscr/rjaf436
Close - Share Icon Share
Abstract
Andersson lesion (AL) is a complication of ankylosing spondylitis (AS), characterized by non-neoplastic destruction of the intervertebral disc and adjacent vertebral bodies. Typically involving the anterior spinal column, AL may be accompanied by posterior column fracture or unfused facet joints abnormalities. We report a case of a young male patient with a long-standing history of AS complicated by AL and ossification of the posterior longitudinal ligament and ligamentum flavum in thoracic spine at the T9 to T10 level. The patient underwent posterior surgical intervention. At a 4-month post-operative follow-up, significant pain relief and marked improvement in lower limb motor function were observed.
Introduction
Andersson lesion (AL), first described by Andersson in the 20th century, is a sterile inflammatory complication observed in the advanced stages of ankylosing spondylitis (AS) [1]. Previous studies have reported a relatively low prevalence, with AL occurring in ~1.5% of AS patients [2]. Despite ongoing research, there remains a lack of consensus regarding the nomenclature and etiology of AL. It is generally considered a chronic manifestation of spinal instability or pseudoarthrosis in the context of a “bamboo spine,” yet its pathological features remain incompletely understood. The management of AS complicated by AL mainly includes both conservative measures and surgical interventions [3, 4]. While thoracic spine involvement is not uncommon in AL, reports of AL in younger patients complicated by ossification of the posterior longitudinal ligament (OPLL) and ligamentum flavum (OLF) are rare [5]. In this study, our medical team presents a case study of a young male patient with AL complicated by AS and significant ossification of both the OPLL and the OLF.
Case description
A 35-year-old male manual laborer presented to the spinal surgery department with a 5-year history of persistent chest pain and a 1-year history of progressive weakness and sensory disturbance in bilateral lower limbs. The patient denied any history of fever and trauma. Physical examination revealed marked kyphosis deformity of the thoracic spine and restricted spinal mobility. A neurological evaluation demonstrated reduced muscle strength in the left lower extremity (Grade II), bilateral positive “4” tests, and positive Babinski signs in both lower limbs. Laboratory investigation revealed HLA-B27 positivity. Inflammatory markers were elevated, with a sedimentation rate of 30 mm/h and a C-reactive protein level of 17 mg/l. Plain radiographs and computed tomography (CT) scans demonstrated the characteristic appearance of a “bamboo spine” that is consistent with advanced AS. A narrowed intervertebral space at T9-T10 vertebrae and prominent ossification of the paravertebral ligaments were observed (Fig. 1A–H). Magnetic resonance imaging (MRI) demonstrated the destruction of the T9-T10 vertebral bodies (Fig. 1I–N).
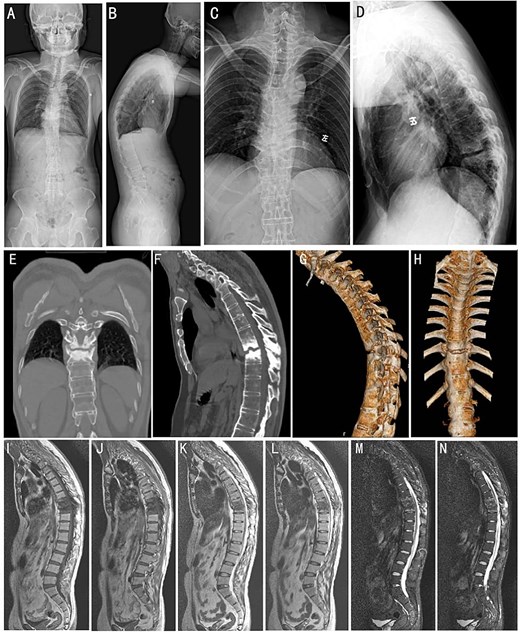
Preoperative imaging studies. (A and B) Full-length orthopantomogram of the spine. (C and D) Anteroposterior and lateral X-ray of the thoracic spine. (E and F) Sagittal and 3D reconstruction CT images (G and H) of the thoracic spine. Whole-spine MRI images of T1-weighted (I and J), T2-weighted (K and L), and short tau inversion recovery sequences (M and N), respectively.
Surgical intervention was performed under general anesthesia. A posterior surgery approach from T8 to T12 was used under intraoperative C-arm fluoroscopy guidance. Pathological analysis of the intervertebral disc tissue confirmed the absence of inflammatory cell infiltration (Fig. 2A–C). Post-operative X-ray and CT imaging confirmed good alignment of the thoracic spine and stable internal fixation (Fig. 2D–I).
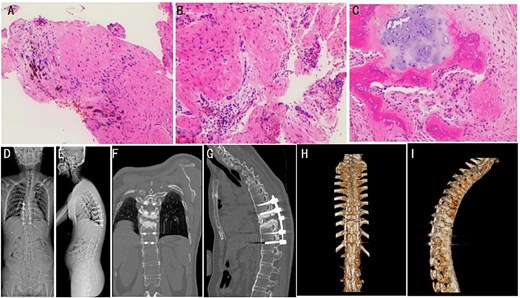
(A–C) Intraoperative pathology specimens obtained for further analysis. (D and E) Post-operative full-length spinal X-ray images. Typical post-operative Sagittal (F and G) and 3D CT reconstruction (H and I).
At a 4-month follow-up, an X-ray imaging confirmed the stable positioning of the instrumentation (Fig. 3A and B). The patient’s muscle strength demonstrated full recovery, with muscle strength in both lower limbs restored to Grade V. After 1 year, CT images showed evidence of bony fusion at the lesion site (Fig. 3C and D). After the 2 years follow-up, X-ray and CT images indicated solid bony fusion (Fig. 3E–H).
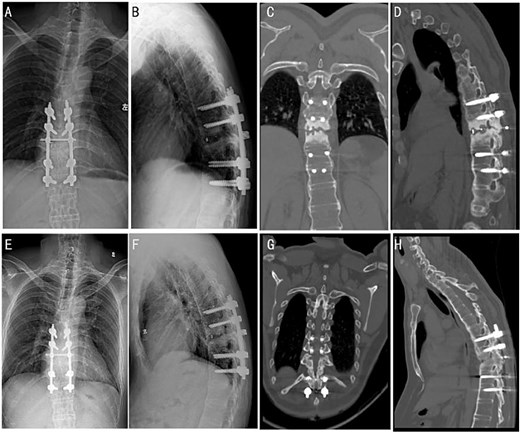
Post-operative follow-up images at different time periods. X-ray images were followed up 4 months after the surgery (A and B). CT images of patients were followed up 1 year after the surgery (C and D). X-ray (E and F) and CT (G and H) images were followed up 2 years after the surgery.
Discussion
AL, first reported by Andersson in 1937, is a complication of AS characterized by destructive lesions involving the vertebral bodies or intervertebral discs [6]. However, despite years of study, its pathogenesis remains controversial. Proposed mechanisms include low-grade infection, inflammation, and mechanical stress. Of these, inflammation and mechanical stress are the most widely accepted explanations among current researchers [7, 8].Clinically, AL most the commonly affects the thoracolumbar junction (T10-L2) due to the high biomechanical stress this area. This stress may predispose the region to microtrauma and fatigue fractures. In the present case, the lesion occurred at T9-T10, supporting the hypothesis that repetitive mechanical stress and local instability contribute significantly to AL pathogenesis.
Imaging features of AL typically reveal destructive changes in the vertebral bodies or intervertebral discs [9]. Conventional radiography has a high rate of missed diagnosis, particularly in mid to late-stage AS. CT imaging offers superior resolution for assessing spinal cord compromise and severity of vertebral or disc destruction [10]. MRI provides further value by differentiating inflammatory and fatty changes in vertebral marrow. These imaging modalities were instrumental in the diagnosis and surgical planning in our case.
To date, no standardized guideline exists for the management of AL. Conservative treatment including medication and plaster [11, 12]. However, conservative measures have limited utility in patients with extensive AL [5]. Nevertheless, severe pain, progressive aggravation of symptoms, and kyphosis deformity still provide essential reference values for surgical treatment [13]. Recently, Zhang et al. [14] demonstrated that minimally invasive spinal surgery (MIS) with posterior instrumentation is a promising treatment option for AL, offering advantages such as reduced intraoperative blood loss and shorter operative time compared to open spinal fusion (OSF). Additionally, Zhang et al. [15] found that in a clinical follow-up of 11 patients with AL who underwent surgical treatment, only one patient experienced post-operative neurologic impairment. However, in the present case, the use of MIS or an anterior surgical approach would have posed significant challenges due to the limited operative field and complex anatomical alterations, making adequate exposure and deformity correction difficult. In contrast, OSF surgery allows for the complete removal of inflammatory tissue and the reliable collection of pathological specimens. Therefore, we selected posterior surgery to achieve thorough spinal canal decompression, which ultimately resulted in favorable outcomes.
Conclusions
Prompt and accurate diagnosis of AL by experienced spinal surgeons can reduce the suffering and financial burdens of the patients. This case provides valuable clinical insight into the diagnosis, treatment, and prognosis of AL. For AL cases with ambiguous anatomical localization, we utilized intraoperative navigation technology to enhance surgical precision and accurate placement of pedicle screws. In summary, our findings provide important guidance for the management of AL combined with AS, particularly when complicated by OPLL and OLF.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This study was supported by the Guangzhou Science and Technology Plan City-School Joint Project (2023A03J0266), the Science and Technology Plan of Guangzhou City Project (202102020108), and Guangdong Second Provincial General Hospital’s Special Fund for the Lifting Project (TJGC-2023009).



