-
PDF
- Split View
-
Views
-
Cite
Cite
Norman A Rendón Mejía, Luis R Ortega Moriel, Ernesto Sandoval Campa, Sofía C G Cristóbal, Carlos R Cervantes Sánchez, Sclerosing peritonitis and peritoneal pseudocyst: a rare cause of surgical acute abdomen in peritoneal dialysis patients—diagnostic and therapeutic insights, Journal of Surgical Case Reports, Volume 2025, Issue 4, April 2025, rjaf244, https://doi.org/10.1093/jscr/rjaf244
Close - Share Icon Share
Abstract
Peritoneal pseudocysts are benign, fluid-filled structures that arise from the accumulation of intra-abdominal fluid, which would typically be reabsorbed by the peritoneum. These pseudocysts form when peritoneal integrity is disrupted by adhesions, often secondary to trauma, surgery, or infection. While intraperitoneal pseudocysts are rare, they are most frequently associated with inflammatory processes, including ventriculoperitoneal shunt complications, catheter-related infections. Sclerosing peritonitis (SP), a rare but serious complication of long-term continuous ambulatory peritoneal dialysis, is characterized by marked thickening of the peritoneal membranes. Histologically, these membranes consist of dense fibrotic connective tissue infiltrated by mononuclear and polymorphonuclear cells. The pathogenesis of SP-associated pseudocysts is thought to involve low-grade infection or localized chronic inflammation. A 59-year-old male on peritoneal dialysis presented to the emergency department with chronic abdominal pain. Computed tomography imaging revealed a large peritoneal pseudocyst. During exploratory midline laparotomy, the pseudocyst was successfully evacuated, and the peritoneal dialysis catheter was removed.
Introduction
Peritoneal pseudocysts (PPs) are rare complications of peritoneal dialysis (PD). These cysts contain fluid and are isolated from abdominal organs. Only six cases were documented between 1985 and 2006, followed by three cases in 2010 and one in 2018 [1]. Also termed peritoneal inclusion cysts, inflammatory peritoneal cysts, or postoperative peritoneal cysts, PPs are benign mesothelial-lined cystic structures within the abdominal cavity [2]. While most abdominal pseudocysts occur in non-nephrological populations—often following ventriculoperitoneal shunt placement for hydrocephalus (incidence: 1%–4.5%) [3]—their etiology in PD patients remains unclear. Proposed mechanisms include chronic inflammatory reactions at the PD catheter tip, triggered by infection, or hypersensitivity [4].
Sclerosing peritonitis (SP), a severe complication of long-term continuous ambulatory peritoneal dialysis, is characterized by markedly thickened peritoneal membranes composed of dense fibrotic connective tissue infiltrated by mononuclear and polymorphonuclear cells [5]. SP typically manifests as partial bowel obstruction and encapsulated bowel loops due to sclerotic peritoneal thickening, alongside clinical features such as abdominal pain, weight loss, and elevated inflammatory markers [6]. We present a 59-year-old male on PD who presented to the emergency department with chronic abdominal pain.
Case presentation
A 59-year-old male with a history of type 2 diabetes mellitus, systemic arterial hypertension, and chronic kidney disease (CKD) (diagnosed in 2003 and progressing to renal replacement therapy 5 years prior to admission) presented to the emergency department. He had undergone PD via an abdominal catheter placed 1 year earlier. The patient reported a 3-month history of intermittent, generalized abdominal pain localized predominantly at the catheter insertion site. The pain was oppressive, rated 8/10 on the visual analogue scale (VAS), and exacerbated during and after PD sessions. Associated symptoms included nausea, vomiting of gastric contents, and reduced fluid inflow/outflow during dialysis. Physical examination revealed a 10 cm diameter, indurated, fixed, non-depressible protuberance at the catheter exit site. Vital signs were within normal limits.
Abdominal computed tomography (CT) demonstrated a large encapsulated cystic mass (9.98 × 12.8 × 23.35 cm) in the abdominal cavity. The lesion adhered to the posterior surface of the anterior abdominal wall, enveloped the dialysis catheter tip, and contained fluid (Fig. 1a and b). Exploratory laparotomy via a midline infraumbilical incision revealed a 20 × 15 cm preperitoneal pseudocyst with dense adhesions to peritoneal tissue and small bowel loops (Fig. 2a and b). The cyst was incised, yielding copious serous fluid, and the entire capsule was excised. The dysfunctional dialysis catheter was concurrently removed. Gross examination identified an irregular peritoneal cyst fragment (14.0 × 18.7 cm) with a pinkish-yellow hue and rubbery consistency, alongside a smaller nodular fragment (4.0 × 3.5 × 2.0 cm). Microscopic analysis revealed adipose tissue with extensive fibrosis and fibroblastic proliferation.
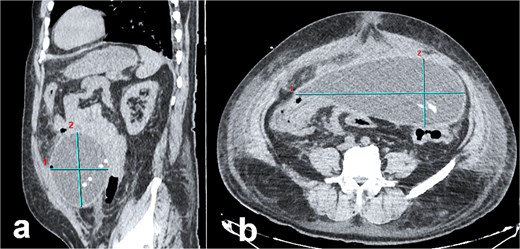
Abdominal CT imaging of peritoneal pseudocyst. (a) Sagittal view: The dialysis catheter tip (arrow) is visualized within the pseudocyst. Note collapsed small bowel loops displaced into the retroperitoneum. (b) Axial view: Encapsulated pseudocyst with thick, enhancing walls (asterisk), and homogeneous serous fluid content. Bowel loops are displaced posteriorly into the retroperitoneum.
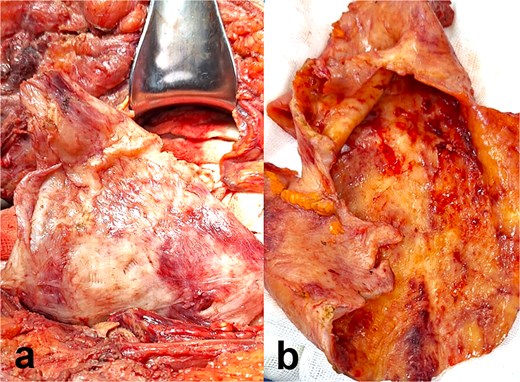
Intraoperative and gross pathological findings. (a) Intraoperative view: The pseudocyst exhibits smooth, hyperemic walls with dense adhesions to the parietal peritoneum (arrowheads) and scattered hemorrhagic foci. (b) Cross-sectional gross specimen: Fibrous capsule with thick, irregular walls (double arrow), and serous fluid within the lumen. Adherent fibrotic tissue is evident at the pseudocyst–peritoneum interface.
The patient resumed enteral feeding (clear liquids) on postoperative Day 2, with return of flatus and bowel movements. By Day 3, he tolerated oral intake and was discharged in stable condition. His recovery was uncomplicated, and haemodialysis was initiated as ongoing renal replacement therapy.
Discussion
Intraperitoneal pseudocyst formation, a rare but serious complication of PD, is exemplified by the case of a 59-year-old male with longstanding type 2 diabetes, hypertension, and CKD progressing to end-stage renal disease. This patient’s clinical trajectory underscores the interplay of chronic inflammation, peritoneal membrane dysfunction, and mechanical stressors inherent to PD. His presentation—marked by a 3-month history of localized abdominal pain (8/10 on VAS), nausea, and impaired dialysate flow—aligns with the nonspecific symptomatology typical of pseudocysts, which often masquerade as intestinal obstruction or chronic peritonitis [4]. The physical finding of a fixed, indurated abdominal mass at the catheter site further highlights the role of localized inflammation in pseudocyst pathogenesis [7].
The patient’s abdominal CT findings—a large encapsulated cystic mass (9.98 × 12.8 × 23.35 cm) enveloping the dialysis catheter tip—illustrate the sequela of disrupted peritoneal integrity. As described in PD literature, pseudocysts arise from aberrant fluid reabsorption due to adhesions or chronic inflammation, often exacerbated by repeated dialysate instillation [7, 8]. The cyst’s adherence to the anterior abdominal wall and small bowel loops mirrors the propensity of these lesions to form within loculated compartments bounded by peritoneal adhesions [2–7, 9, 10]. Histopathological analysis, revealing fibrous collagenous tissue and fibroblastic proliferation without epithelial lining, corroborates the role of chronic inflammation and macrophage-derived IL-1 in driving fibrogenesis [8]. These findings align with the proposed mechanism whereby recurrent peritoneal insults (e.g. catheter-related infections) incite fibroblast activation and collagen deposition, culminating in cyst wall formation [3, 8].
This case reinforces the necessity of surgical intervention for large or symptomatic pseudocysts. The successful excision of the 20 × 15 cm pseudocyst via midline laparotomy, alongside catheter removal, underscores the importance of complete cyst resection to mitigate recurrence, as advocated by Costa et al. [11]. The patient’s rapid postoperative recovery—resuming enteral feeding by Day 2 and transitioning to haemodialysis—highlights the efficacy of timely surgical management in preventing fatal complications like SP or bowel obstruction [6, 11]. Notably, the pseudocyst’s serous content contrasts with the hemorrhagic or purulent fluid described in non-PD cases [12], suggesting PD-associated pseudocysts may follow a distinct inflammatory trajectory [13].
The reported 1%–4% incidence of catheter-associated pseudocysts [6, 7, 9, 10] underscores the need for vigilance in PD patients with risk factors such as prior abdominal surgery or recurrent peritonitis—both pertinent to this patient. While larger dialysate volumes in PD may reduce loculation risk compared to ventriculoperitoneal shunts [6, 7, 9, 10], this case illustrates that chronic inflammation and mechanical trauma from long-term catheter use remain potent drivers of pseudocyst formation.
Conclusion
PPs, an exceedingly rare complication of long-term PD, are sparsely documented in clinical literature. Although their pathogenesis remains poorly defined, current evidence suggests they arise from chronic inflammation at the PD catheter tip, driven by recurrent infection or hypersensitivity reactions. Clinically, patients often present with nonspecific abdominal symptoms, including localized pain, distention, nausea, and vomiting. Definitive management necessitates complete surgical excision of the pseudocyst to minimize recurrence risk, as residual cystic tissue may perpetuate inflammatory cascades or mechanical complications.
Conflict of interest statement
None declared.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
Written and verbal consent were obtained from the patient for publication of this case, clinical findings, and related images. Written consent is available to the Editor-In-Chief upon request.
References
- peritoneal dialysis
- acute abdomen
- computed tomography
- catheter-related infections
- chronic abdominal pain
- adhesions
- connective tissue
- emergency service, hospital
- laparotomy
- tissue membrane
- neutrophils
- peritoneal dialysis, continuous ambulatory
- peritonitis
- surgical procedures, operative
- infections
- abdomen
- diagnosis
- diagnostic imaging
- peritoneum
- surgery specialty
- ventriculoperitoneal shunt
- pseudocyst
- pseudotumor
- chronic inflammation
- laparotomy, exploratory
- intraperitoneal infusion
- trauma surgery
- peritoneal dialysis catheter
- personal integrity



