-
PDF
- Split View
-
Views
-
Cite
Cite
Trần T Sơn, Phạm T Việt Dung, Tạ T Hồng Thuý, Phạm K Nhật, Mitsuru Sekido, Phan T Nghĩa, Extended DIEP flap with bilateral anterior fascia based on unilateral perforator for lower extremity reconstruction, Journal of Surgical Case Reports, Volume 2025, Issue 4, April 2025, rjaf201, https://doi.org/10.1093/jscr/rjaf201
Close - Share Icon Share
Abstract
Complex soft tissue defects in the lower limb pose significant challenges for surgeons, especially when extensive coverage is needed. Free flaps are preferred for their reliable soft tissue, with the deep inferior epigastric perforator (DIEP) flap being particularly advantageous in middle-aged women due to its ample tissue and the ability to perform abdominoplasty at the donor site. However, its bulkiness can be an issue for lower limb reconstruction. We present two cases where the DIEP flap was used for lower limb reconstruction with immediate flap thinning. By employing a unique thinning method based on the vascular connection between perforators, we successfully extended the flap's use without needing to anastomose the pedicles. All flaps were well-vascularized with no complications, demonstrating that maintaining the anterior fascia between perforators optimizes the DIEP flap for reconstructive purposes.
Introduction
To date, the deep inferior epigastric perforator (DIEP) flap has been considered the first choice for autologous tissue-based breast reconstruction after total mastectomy. This flap has several advantages, including a substantial volume of skin and tissue, reliable vascular supply, and reduced morbidity at the donor site [1]. Anatomical and clinical studies have identified the blood supply range of the perforators for this flap, particularly in perfusion zone IV of the Holm and Hartrampf classifications, which is considered an unsafe area [2, 3]. Many researchers have attempted to expand the safe blood supply range of the DIEP flap by combining two perforators or by pre-expanding the skin. While these methods are effective in preventing necrosis in perfusion zone IV, they tend to prolong the operation time [4, 5].
Complex soft tissue defects in the lower extremities always pose a challenge for surgeons. The reconstructive material must be sufficiently large, possess a good blood supply, and adequately cover exposed tendons and bones while also considering aesthetics at both the donor and recipient sites [6]. A DIEP flap can be a suitable option, especially for adult female patients. In this report, we will share our experience using the free DIEP flap for reconstructing lower limb soft tissue defects and detail our technique for extending the flap's blood supply.
Case series
From March 2017 until December 2018, two female patients presented with lower limb soft-tissue defects caused by a traffic crush injury at our department. All soft-tissue defects with exposed tendons and bones were initially debrided in the orthopedic department before the patients were transferred to our department. Two patients underwent reconstruction with free DIEP flaps. The follow-up time ranges from 2 to 6 years.
Surgical technique
The DIEP flap is harvested for lower limb reconstruction, similar to breast reconstruction. Preoperatively, we marked the perforators with a hand-held Doppler. We proceeded with the surgery only after identifying two perforators at the same level. The design of the transverse skin flap was based on the size of the defect. The incision begins at the inferior border of the flap and is typically positioned similarly to that in an abdominoplasty. Dissection is carried out from lateral to medial above the deep fascia until suitable perforators are located. The upper incision is created to reduce extreme tension for primary closure while still accommodating the lesion size; generally, the flap width is ⁓12–13 cm. The two largest perforators were identified on both sides of the anterior rectus sheath. We dissected a fascial island connecting the two perforators. A fascial island measuring ⁓5 cm in width and 12 cm in length was dissected from the rectus abdominis muscle layer, with both perforators located in one intramuscular septal row. Then, we selected the one with adequate outflow. The DIEP was dissected up to the origin in the femoral vessels, which provided a pedicle length of 14–15 cm. A second perforator was located nearby in the same septal row, it would then be cut and ligated. The flap is thinned while the pedicle remains intact. Adipose tissue is removed subfascially, dissecting from the periphery toward the center of the flap, limited by the fascial island between the two perforators. The flap was then transferred to the recipient site. End-to-end micro-anastomosis is performed on the anterior tibial artery. A dose of 5000 units of heparin is administered intravenously during the anastomosis. The donor site is then closed primarily. Postoperatively, flap reflux should be checked every 3 h. The amount of fluid draining through the drain is also monitored, and the drains are typically removed on day 7. Antibiotics are administered for 3 days.
Case 1
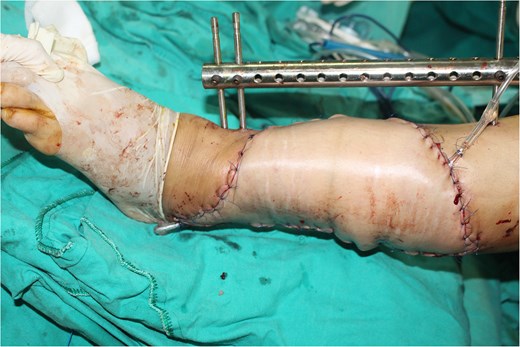
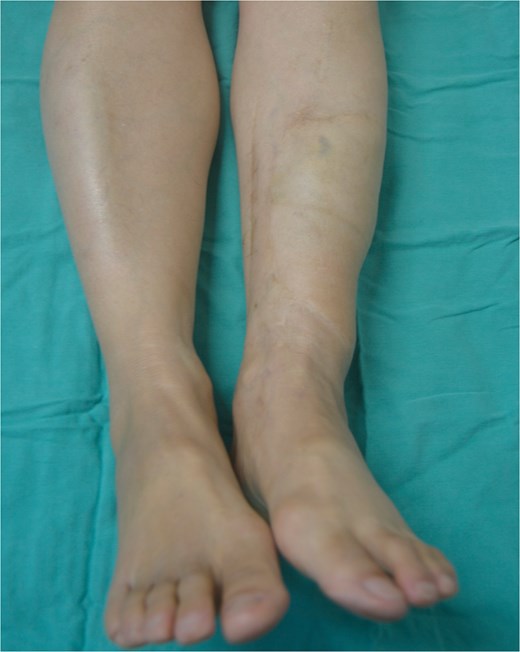
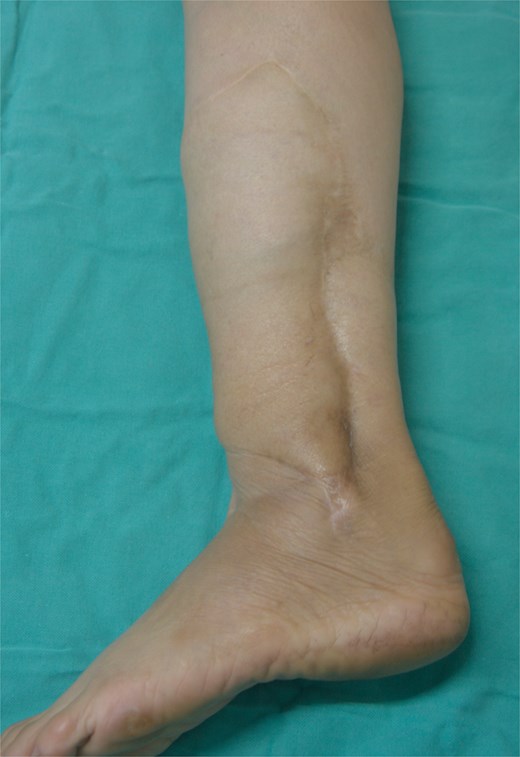
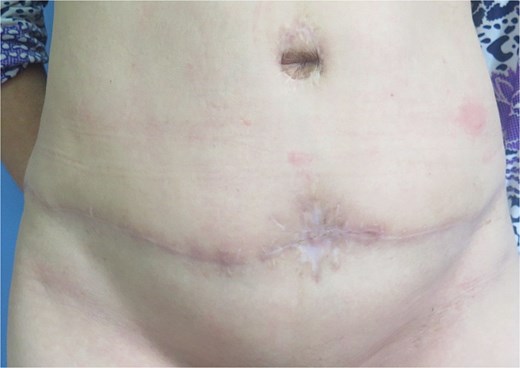
A 49-year-old female maintained an open tibia and fibula fracture with a major degloving injury of the lower two-thirds of the right leg. The orthopedic team debrided the wound, and the tibia was stabilized using external fixation, while the fibula was stabilized with internal fixation (Fig. 1). One week later, the patient was transferred to our department to address the soft tissue defect. The skin defect measured 15 × 24 cm. Using a handheld Doppler, we identified two large perforators at the same level on each side of the flap (Fig. 2). We decided to harvest a DIEP flap measuring 14 × 26 cm. The left deep inferior epigastric pedicle was used for the flap. We preserved a fascia island measuring 5 × 12 cm between the two perforators and secured the right perforator (Fig. 3). The flap was thinned from 25 mm to 15 mm on all sides except the area where the fascia was left intact (Fig. 4). The flap was then transferred to cover the skin defect. We anastomosed the pedicle to the anterior tibial artery and two veins end-to-end. The flap was positioned around the anterior and lateral aspects of the distal two-thirds of the lower leg (Fig. 5). The donor site was closed directly, similar to an abdominoplasty. The flaps survived well, with no complications observed. Twelve months after the surgery, a liposculpture procedure was performed on the flap. The appearance of the flap and the scar on the donor site 6 years postoperatively are shown in Figs 6–8.
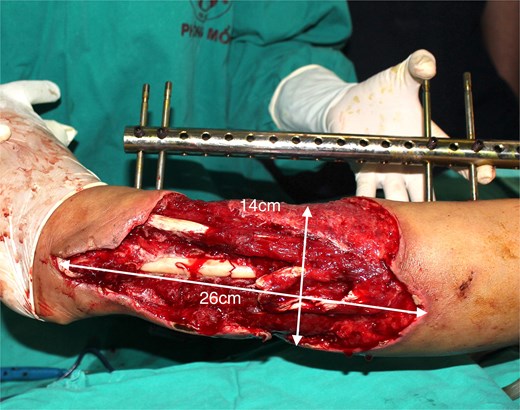
Patient 1. A 49-year-old female. Complex injury of the lower two-thirds of the right leg.
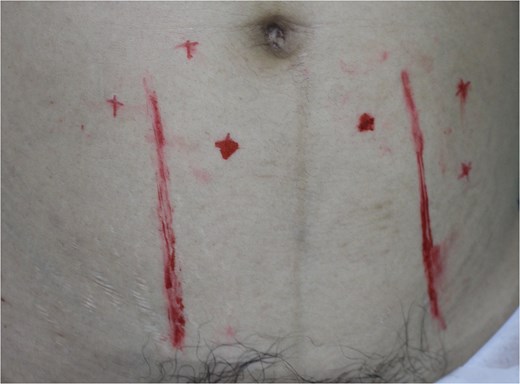
The perforators preoperatively were marked with a hand-held Doppler.
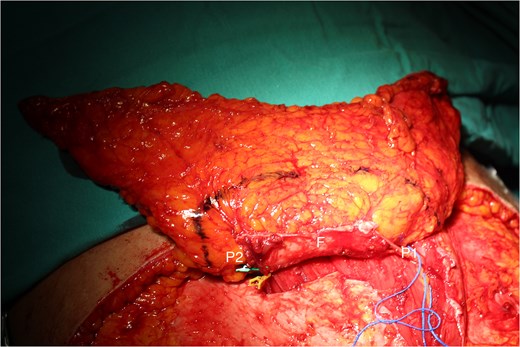
The DIEP flap with the size of 14 × 26 cm was dissected with two perforators (P1 and P2), and the rectus abdominis muscle fascia connecting the two perforators (F) was retained within.
The flap was thinned and removed from the donor site to cover the defect.
Case 2
A 53-year-old female sustained large defects of the skin and soft tissue of the left foot and ankle due to a traffic accident. The heel was compromised, exposing the Achilles tendon. The patient was then admitted to our department, where we observed a lesion measuring 15 × 25 cm accompanied by a wound infection. We used vacuum-assisted-closure for a duration of 5 days. A 14 × 30 cm DIEP flap was harvested to reconstruct the soft tissue defect. The left pedicle was utilized, with the fascial island measuring 5 × 12 cm. The flap was thinned primarily to a thickness of 15 mm. The flap was successfully revascularized through end-to-end anastomoses to the anterior tibial artery and its concomitant veins. The flap demonstrated good survival, with no complication observed. The donor site was primarily closed.
Discussion
Complex soft tissue injuries in the lower limbs caused by factors such as burns, post-tumor resections, and especially trauma present significant challenges for plastic surgeons. In traumatic injuries, soft tissue defects can involve skin degloving, soft tissue necrosis, exposed tendons and bones, and even exposed joints, making treatment particularly difficult, especially when infections are present [7]. Surgeons face the challenge of ensuring adequate coverage for extensive soft tissue defects while maintaining a rich blood supply, appropriate contours and function, and aesthetic considerations. Microsurgical flaps are typically the preferred method for reconstructing these defects, as they help restore function and appearance early on, thanks to their abundant blood supply and effective resistance to infection [6]. Various types of free myocutaneous flaps, such as the latissimus dorsi myocutaneous flap, gracilis myocutaneous flap, fasciocutaneous lateral arm flap, and anterolateral thigh flap, meet the requirements for large flap volume, with long vascular pedicles for coverage [8, 9]. However, the choice of flap can often be influenced by concerns over donor site morbidities, in addition to surgeon experience. The DIEP flap, first described by Koshima et al., has become a popular and reliable option for autologous breast reconstruction following mastectomy [10]. Due to its large tissue volume, rich vascular supply, and versatility from varying blood sources, the DIEP flap is indicated for reconstructing various body areas [11, 12]. In lower limb reconstruction, the vertical or oblique DIEP flap variations enhance the ability to mobilize the flap area, particularly in male or pediatric patients [13, 14]. Combining two blood supplies from different perforators and employing chimeric fabricated flaps can effectively extend the blood supply DIEP flap to cover large area limb defects [15, 16].
In anatomical studies, the lateral and medial perforators from DIEA dominate the lower abdominal wall. Each perforator holds a unique tissue territory (perforasomes), and these perforasomes are independent in physiological situations. In the course from muscle to skin, the perforators divide into four plexuses: prefacial, suprafacial, subcutaneous, and subdermal plexus. In the perforasomes, the plexuses are connected through the direct and indirect linking vessels and play a critical role in flap perfusion. The perforasomes are themselves linked by communicating branches. After coursing in the rectus muscle fibers, the perforators directly pierce or travel along the anterior sheath of the rectus and then course through the subcutaneous fat [17]. The fascial plexus is related to the deep fascia and only exists in the fasciocutaneous flap. This layer has provided an alternative pathway beside the perforator [18]. The subcutaneous plexus lies in the superficial fascia and consists of linking vessels which connect to the surrounding perforasomes. Direct flow through the linking vessel of the fascial and subcutaneous plexuses communicates directly with an adjacent perforator. These networks supply blood mainly to the fascia and the deep adipose tissue. The indirect flow through the subdermal plexus takes the blood to the superficial fat and the skin [19]. In clinical or cadaveric studies of the DIEP flap, the fascial layer and fascial plexus system are removed, at this point, the blood supply to the flap is mainly provided by the subcutaneous and subdermal plexus systems. Large flaps can be harvested based on a single perforator; in this situation, choked vessels are communicated to adjacent perforators via direct and indirect linking vessels. Hyperperfusion of a single perforator can attach multiple adjacent perforasomes, and the extended areas are called the dynamic territory of the flap [20]. In the DIEP flap, there is a single perforator on one side; zone I is the tissue territory where the perforator's skin entry site is. Zone II and III, the safe areas, are adjacent to Zone I. Zones II and III are mainly due to the opening of the choked vessels, which leads to an expansion of the dynamic zone. Zone IV is located in the distal region on the opposite side, where direct and indirect choked vessels are not opened, this area is unsafe [2, 3, 19]. The single-pedicle flap is considered a conventional flap in breast reconstruction or for coverage of soft tissue defects on the body. Many authors have proposed technical improvements to increase the blood supply to zone IV. Dissecting more perforators on the same deep inferior epigastric artery axis has been shown to make no significant difference to the safety of zone IV but significantly increases the dissection time and the risk of microvascular injury.
The subcutaneous and subdermal vascular systems are crucial to the blood supply to the skin flap. Notably, the fascial vascular system plays a significant role in connecting the two perforators on either side of the abdominal wall. When this fascial layer is maintained, the fascial and subfascial plexus systems allow for better communication between the two perforator systems, particularly when the perforators lie at the same level. Therefore, the blood supply to this area exceeds the ipsilateral adjacent area. We retained the fascial layer between the two perforators, which was essential for expanding the flap's blood supply area. In this context, zone III, adjacent to zone I according to Holm's classification, receives more blood than zone II. We have designated this area as zone IIa, while Holm’s zone II is referred to as zone IIb. The previous zone IV is now called zone III (Fig. 9). In addition to enhancing the anastomosis between the two perforators, we performed primary thinning by removing the entire layer of fat beneath the superficial fascia. This step reduced the flap's volume while preserving the vascular system within the superficial fascia. Blood supply was successfully extended to the distal end of the flap, as indicated by bleeding at the flap edges before detachment and after microsurgical anastomosis. Both patients had large skin flaps with lengths between 24–30 cm, and we did not require anastomosis or the use of two vascular pedicles. This approach minimized the dissection of the contralateral vascular pedicle and reduced the time needed for anastomosis. With this technique, we have successfully applied it not only to reconstruct lower limb defects but also to neck and breast reconstructions following breast cancer surgery.
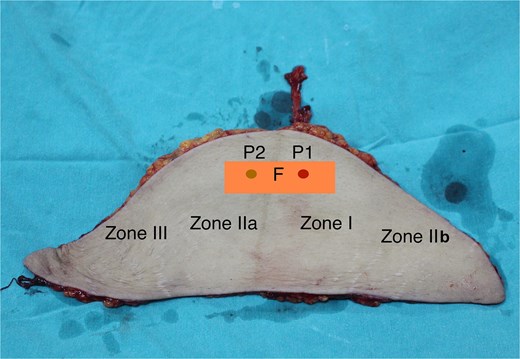
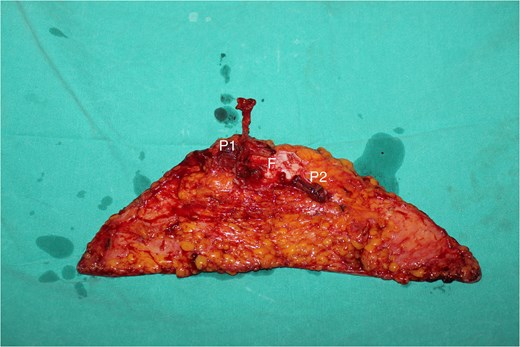
The perfusion zones in the flap with the fascial strip (P1: The pedicle’s perforator; P2: Perforator in the contralateral side, was ligated; F: The inter perforator fascia with 4 × 12 cm size).
However, there are limitations to this technique: it cannot be applied to male patients, children, cases where only one perforating vessel is detected on one side of the abdominal wall, or instances with multiple small perforating vessels.
Conclusion
Extending the vascularity of the DIEP flap by maintaining the fascia between the two perforators is an appropriate reconstructive option for female patients with large soft tissue defects in the lower extremities.
Conflict of interest statement
None declared.
Funding
None declared.



