-
PDF
- Split View
-
Views
-
Cite
Cite
Liman Zhang, Jie Yang, Qiang Wang, Lili Wang, Shuzhen Su, Lifang Wang, Shiyuan Li, Transvaginal repair of rectocele for obstructed defecation syndrome: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 4, April 2025, rjaf191, https://doi.org/10.1093/jscr/rjaf191
Close - Share Icon Share
Abstract
Rectocele (RC), defined as the protrusion of the anterior rectal wall into the vaginal lumen, is a significant cause of obstructed defecation syndrome (ODS). This case report describes a 60-year-old female patient with chronic constipation diagnosed with Grade III RC-induced ODS after excluding organic bowel diseases and slow-transit constipation. The patient underwent transvaginal repair of the RC, resulting in significant clinical improvement. Combined with a review of the literature, this article discusses the diagnostic criteria, surgical indications, and treatment strategies for RC, providing valuable insights for clinical practice.
Introduction
Rectocele (RC) is a condition where the anterior and inferior part of the rectum protrudes into the vagina, forming a pouch [1]. Fecal retention within this pouch during defecation leads to difficulty in bowel movements, making it one of the important causes of obstructed defecation constipation. On digital rectal examination, a distinct indentation protruding into the vagina can be palpated in the lower rectum. Defecography can clearly display the depth and extent of the protrusion, classifying RCs into three grades: I, II, and III [2]. Grades I and II RCs are typically managed conservatively, while surgical repair is considered for patients with Grade III RC who have been confirmed to have obstructed defecation constipation [3]. This case report describes the process of treating a patient with obstructed defecation constipation using transvaginal repair, demonstrating the effectiveness of this surgical approach.
Case report
A 60-year-old female presented with a 3-year history of progressively worsening defecatory dysfunction characterized by prolonged straining (⁓15 min per episode), incomplete evacuation, and reliance on manual vaginal pressure to facilitate stool expulsion. Despite daily bowel frequency (twice per day), stools remained soft-formed but impacted at the anal verge. Physical examination in the lithotomy position revealed no anal fissures or sphincteric abnormalities (no evidence of sphincter hypotonia or hypertonia on digital rectal examination). A palpable rectovaginal bulge was identified during digital assessment, while proctoscopy demonstrated no intraluminal lesions. Routine laboratory investigations—including complete blood count, fasting glucose, hepatic/renal function panels, coagulation studies, electrolyte profiles, infectious serologies, and electrocardiography—were unremarkable. Colonoscopy excluded organic colorectal pathology (Fig. 1).
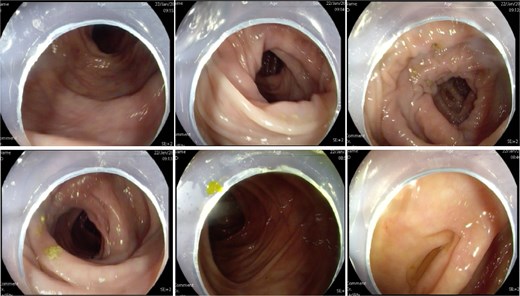
Colonoscopy showed no abnormal lesions in the bowel after admission.
Colonic transit time assessment was performed using radiopaque markers. At 24 h post-ingestion, 19 markers were retained within the colon, with complete clearance observed at 48 and 72 h, confirming normal colonic motility (Fig. 2). Defecography demonstrated a Grade III RC, characterized by a maximal depth of 76 mm during straining and persistent barium retention post-evacuation, consistent with obstructed defecation syndrome (ODS) (Fig. 3).
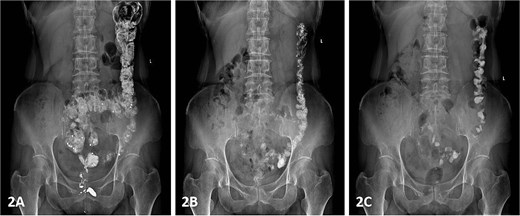
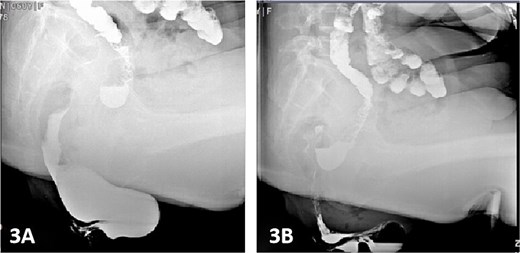
Following exclusion of organic bowel disorders and colonic dysmotility, combined with the patient's history of manual vaginal pressure-assisted defecation and defecographic confirmation of a 76-mm RC, the diagnosis of RC-induced ODS was established. Preoperative gynecological consultation ruled out concurrent vaginitis or pelvic organ prolapse. Bowel preparation included vaginal irrigation with normal saline (2–3 times daily) for 2 days preoperatively, oral polyethylene glycol-electrolyte lavage 24 h prior to surgery, and preoperative morning cleansing enema.
Under spinal anesthesia, the patient was placed in the lithotomy position, and the vaginal and rectal areas were thoroughly disinfected using povidone-iodine. A 1:500 epinephrine-saline solution (10–20 mL) was injected into the submucosal layer of the posterior vaginal wall to facilitate tissue dissection and minimize bleeding. A horizontal incision measuring ⁓8 cm in length was created at the vaginal mucocutaneous junction (hymenal ring). Dissection was performed along the rectovaginal septum, extending to 1 cm above the apex of the RC pouch. The RC sac was fully exposed by dissecting laterally to the levator ani muscles. The levator ani muscles were then approximated using interrupted 7–0 silk sutures to reinforce the rectovaginal septum and eliminate the RC pouch. Excess vaginal mucosa was resected according to the degree of rectal protrusion and posterior vaginal wall laxity, and the vaginal mucosa was closed with interrupted 7–0 silk sutures. A Foley catheter was inserted, and the vaginal cavity was packed with iodine-impregnated gauze to maintain hemostasis and support the repair (Fig. 4).
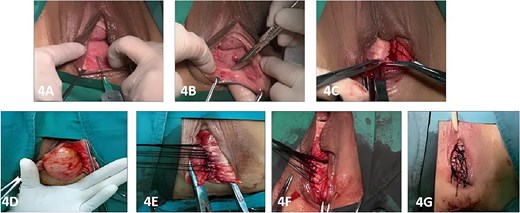
Postoperatively, the patient was administered intravenous cefuroxime and metronidazole for 3 days as prophylactic antimicrobial therapy. The iodine-impregnated vaginal gauze pack and indwelling urinary catheter were removed 24 h after surgery. Daily vaginal dressing changes were performed, involving thorough wound disinfection with povidone-iodine. In cases of excessive vaginal discharge, additional irrigation with normal saline was conducted. The interrupted silk sutures on the posterior vaginal wall were removed during a follow-up visit 2 weeks postoperatively. At 15 days postoperatively, following suture removal, repeat defecography demonstrated a residual RC depth of 11 mm, with complete resolution of the patient’s defecatory dysfunction (Fig. 5). During the 24-month postoperative follow-up period, the patient maintained normal defecatory function with no recurrence of obstructive symptoms. However, repeat defecography was not performed due to the patient’s personal preference.
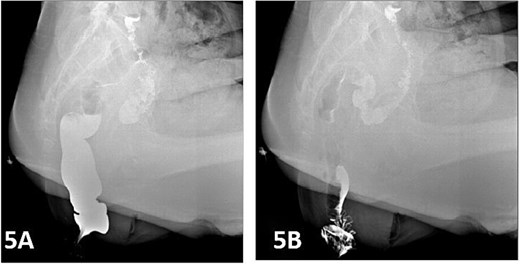
Postoperative defecography at 15 days, the image demonstrates a residual RC depth of 11 mm.
Conclusion
Functional constipation is categorized into three distinct subtypes: slow-transit constipation (STC), ODS, and mixed constipation (MC). ODS constitutes the most prevalent subtype, accounting for ⁓60% of cases, with RC being a frequently identified etiological factor [4]. The affected patients commonly exhibit symptoms including excessive straining during defecation, persistent sensations of anal obstruction and pelvic heaviness, incomplete rectal evacuation, and dependence on manual maneuvers such as digital fecal extraction or perineal/vaginal pressure to facilitate stool expulsion [5]. The consequences of chronic constipation are manifold, not only severely compromising quality of life but also predisposing patients to cardiovascular and cerebrovascular events. Furthermore, iatrogenic rectal mucosal injuries secondary to repeated digital interventions are frequently observed in clinical settings.
RC predominantly affects middle-aged and elderly women, with a reported prevalence ranging from 30% to 50%.The vaginal wall is primarily composed of fascial tissue, incorporating smooth muscle fibers, collagen, elastin, and neurovascular structures. The rectovaginal septum, anatomically predisposed to structural weakness, undergoes progressive deterioration due to age-related hormonal changes, pregnancy, and parturition [6], resulting in pelvic floor connective tissue laxity and excessive anterior displacement of the rectovaginal septum into the vaginal lumen. This pathological stretching prevents complete fascial recovery, ultimately leading to RC formation. The diagnostic evaluation primarily involves digital rectal examination combined with defecography. While magnetic resonance defecography has gained clinical utility, its diagnostic accuracy is limited by the supine positioning of patients, which fails to replicate the gravitational forces essential for dynamic assessment [7, 8]. Consequently, conventional defecography remains the gold standard for quantifying RC severity and establishing surgical indications.
It is proposed that RC represents a structural anatomical variant rather than a pathological entity, given that a significant proportion of women with RC remain asymptomatic [9]. In cases of mild to moderate RC associated with ODS, first-line management includes conservative measures such as dietary modification, pharmacological laxatives, pelvic floor muscle training (Kegel exercises), and biofeedback rehabilitation [10]. Surgical intervention is reserved for patients with moderate to severe RC who have not responded to conservative management and only when meeting specific selection criteria, as it is a definitive therapeutic option [11]. Surgical candidates are limited to those with clinically significant RC-related ODS, where the aim of the procedure is to reinforce the rectovaginal septum, eliminate the fascial defect, and restore normal pelvic anatomy. The institutional criteria for surgical intervention include: Firstly, the RC must measure at least 31 mm in depth, as evidenced by post-defecation barium retention. Secondly, colonic motility disorders must be excluded, with the underlying RC-induced ODS confirmed. Thirdly, symptoms must persist despite a minimum of six months of conservative therapy. Finally, dependence on manual vaginal or perineal pressure for defecation is a requirement. It is notable that the requirement for digital assistance demonstrates particular prognostic value in predicting surgical outcomes.
A plethora of surgical approaches are available for RC repair, including transanal, transvaginal, perineal, and transabdominal techniques [12]. Transanal procedures, particularly those utilizing stapling devices such as the Stapled Transanal Rectal Resection (STARR) and Transtar techniques, have gained popularity among colorectal surgeons. However, it is critical to recognize that RC fundamentally represents a herniation of the anterior rectal wall through a deficient rectovaginal septum, with the primary pathology being the structural weakness of the rectovaginal septum rather than intrinsic rectal disease [13]. Consequently, the primary surgical objective should be anatomical restoration through reinforcement of the rectovaginal septum and augmentation of rectal support [14]. Conversely, transanal approaches such as STARR and Transtar are constrained to the resection and stapling of redundant rectal mucosa [15]. The inherent elasticity of rectal mucosal tissue renders these procedures susceptible to recurrence, as the mucosa may progressively herniate through the persistent defect in the rectovaginal septum.
It is important to note that chronic constipation is frequently associated with comorbid psychological disorders or systemic conditions, with purely anatomical abnormalities accounting for only a minority of ODS cases. Surgical intervention is most beneficial for this select subgroup, as conceptualized by the “iceberg theory” [16]. Following RC repair, for example, the underlying contributing factors that include psychological disturbances, endocrine dysfunction, and metabolic disorders often become more clinically apparent. Consequently, in patients with suboptimal surgical outcomes, a comprehensive evaluation and targeted management of these latent factors are essential. This case report emphasizes anatomical restoration and early functional recovery, acknowledging that chronic constipation often involves multifactorial interactions (e.g. psychological or metabolic comorbidities). While these factors were not systematically analyzed here, their potential impact on long-term outcomes underscores the need for integrated bio-psycho-social evaluations in future studies.
In the present case, transvaginal repair achieved successful resolution of Grade III RC-induced ODS, with marked improvement in defecatory function. Supported by current literature, it can be concluded that transvaginal repair represents an effective surgical strategy, particularly for moderate to severe RC. This case report documents the anatomical restoration (residual RC depth: 11 mm) confirmed by 15-day postoperative defecography, along with functional improvements observed at both short-term (15-day) and extended (24-month) follow-up. While these findings highlight the procedural success, long-term outcomes such as recurrence rates and sustained functional stability require validation through prospective cohort studies. Multicenter studies with ≥3-year follow-up periods are strongly recommended to establish robust evidence. Moreover, the transvaginal approach was selected for this Grade III RC due to its capacity to directly address rectovaginal septum defects. Comparative analyses of other techniques (e.g. transanal procedures) and their respective indications necessitate larger-scale controlled trials. Nevertheless, rigorous patient selection based on established surgical criteria and systematic postoperative care remains paramount for optimizing outcomes. Further investigation through large-scale, prospective studies with extended follow-up periods is warranted to validate the long-term efficacy and safety profile of this technique.
Acknowledgements
The authors gratefully acknowledge the invaluable support provided by the Medical Records Department of Shijiazhuang Traditional Chinese Medicine Hospital in supplying case data.
Conflict of interest statement
None declared.
Funding
None declared.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.



