-
PDF
- Split View
-
Views
-
Cite
Cite
Ajit Khadga, Mahesh Bahadur Adhikari, Bipin Maharjan, Ravi Kiran Gautam, Prashant Mishra, Birodh Basnet, Deepak Kumar Yadav, Shoshan Acharya, Unicentric retroperitoneal Castleman’s disease in young Nepalese women, Journal of Surgical Case Reports, Volume 2025, Issue 2, February 2025, rjaf086, https://doi.org/10.1093/jscr/rjaf086
Close - Share Icon Share
Abstract
Castleman disease (CD) is a rare lymphoproliferative disorder of uncertain etiology, most commonly affecting the chest and neck, with retroperitoneal involvement being exceptionally rare. It can present as either unicentric or multicentric disease, with the unicentric form typically affecting younger individuals. Due to its rarity and overlapping features with conditions like non-Hodgkin lymphoma and paraganglioma. CD is often misdiagnosed, making immunohistochemical analysis crucial for accurate diagnosis. We report a rare case of retroperitoneal CD in a 23-year-old female referred to our hospital after a CT scan revealed a retroperitoneal tumor anterior to the lower pole of her left kidney at the L3-L4 vertebral level. An initial core biopsy suggested paraganglioma; however, following laparoscopic tumorectomy, histopathological analysis confirmed Castleman disease of the hyaline vascular and plasma cell type.
Introduction
Angiofollicular lymph node hyperplasia, or Castleman’s disease (CD), is a rare neoplastic disease first identified in 1956 by Castleman et al. [1]. CD most commonly affects the mediastinum (63%), followed by the abdomen (11%), retroperitoneum (7%), and axilla (4%) [2]. Pathologically, it can be classified as hyaline vascular type, plasma cell type, mixed type, and human herpesvirus (HHV)-8-associated CD [3]. Clinically, it manifests as the more common unicentric (localized or unifocal) castleman disease (UCD) and the less common multicentric (generalized or multifocal) CD (MCD) [4]. CD usually develops in most patients before reaching 30, although the age range is broad. Localization of the CD in the retroperitoneum is rare. Diagnosis in this setting is difficult, and most cases are diagnosed based on a postoperative pathological examination. The present study reported a rare case of UCD located in the retroperitoneum, which was completely resected by laparoscopic surgery with limited bleeding and an inadvertent left ureteric injury on a retrograde pyelogram.
Case presentation
A 23-year-old female was referred for an asymptomatic abdominal mass, with mild diarrhea and discomfort for one month. On examination, a non-tender, immobile, smooth, firm-to-soft mass was palpable in the left hypochondrium, with no peripheral lymphadenopathy. Laboratory tests from another center showed a mildly elevated metanephrine creatinine ratio (351.85; N = 0–300), a high non-metanephrine creatinine ratio (496; N = 0–400), anemia (Hb: 8.0 mg/dl), and low mean corpuscular volume (63.7).
Contrast-enhanced CT revealed an 8 × 5.4 × 5.4 cm, well-defined, intensely enhancing retroperitoneal mass anterior to the left kidney, with an arterial supply from the left second and third lumbar arteries. Marked soft tissue stranding suggested a neurogenic tumor, likely an extra-adrenal pheochromocytoma or paraganglioma (Fig. 1a and b). A core biopsy from another center also suggested paraganglioma.
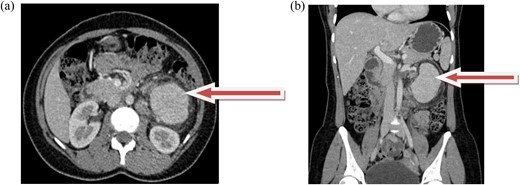
(a and b) axial and coronal computed tomographic scans, respectively, show intensely enhancing round to oval soft tissue density mass in the left retroperitoneal area anterior to the left kidney.
Laparoscopic transperitoneal tumorectomy was performed. During the transperitoneal approach, the tumor was observed anterior to the left kidney (Fig. 2a and b).
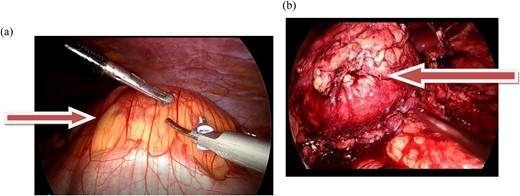
(a and b) The images show the laparoscopic view of a left retroperitoneal mass.
Inadvertently, the left ureter was clipped and cut, mistaking for vessels supplying the tumor. On the retrograde pyelogram there was a contrast extravasation suggesting ureteric injury. A ureteric injury was recognized, and laparoscopic assisted open repair was done. The patient recovered well following the operation and was discharged on the sixth postoperative day.
Pathological findings
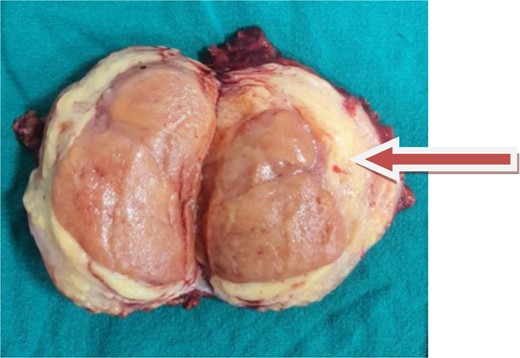
A soft encapsulated mass measuring 12.0 × 8.0 × 1.0 cm with tannish orange cut surfaces was identified in a surgical tissue specimen. The tumor was 2 cm away from the capsule (Fig. 3).
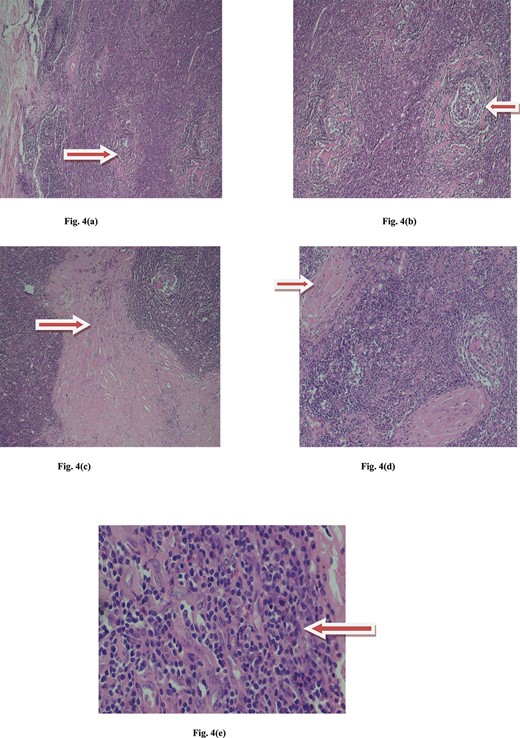
Microscopic view of Castleman disease. (a) Multiple lyphoid follicule with maintained architecture. (b) Interfollicular hypervascularity with sclerotic blood vessels. (c) Interfollicular collagenous band. (d) Sclerotic vessels with onion skin pattern. (e) Lymmmphocytes, eosinophils, plasma cells and histiocytes.
The immunohistochemistry results show a reactive lymphoproliferative pattern with CD3, CD20, CD10, BCL2, KI67, and CD23. These markers suggest a normal immune reaction with active B-cell and T-cell involvement. Polytypic plasma cells are identified by CD138, Kappa, and Lambda, confirming no monoclonal proliferation. The presence of 30 IgG4-positive cells per high-power field in a single focus suggests possible IgG4-related disease. CD30 highlights activated immune cells (immunoblasts), and HHV8 testing is negative, ruling out viral conditions like Kaposi's Sarcoma.
At the 6-week follow-up, the double-J stent was successfully removed. By the 1-year follow-up, the patient reported no symptoms, indicating a positive recovery outcome.
Discussion
The patient presented to our institute with a symptomatic retroperitoneal mass after consulting multiple hospitals, each suggesting different possible differential diagnoses. Primary retroperitoneal tumors are often challenging to diagnose due to their deep location and are typically identified only when they grow large enough to cause compressive symptoms or become palpable during an abdominal examination.
Among primary retroperitoneal tumors, one-third are sarcomas [5]. Liposarcomas comprise 70% of retroperitoneal sarcomas and can often be differentiated on cross-sectional imaging on the basis of fat distribution [6]. CD is rarely included within differential diagnosis regardless of presentation. The decision to biopsy retroperitoneal masses preoperatively remains controversial. Core-needle biopsy is often superior to fine needle aspiration to obtain appropriate specimens for pathologic evaluation, to differentiate variant histologic subtypes of mesenchymal tumors and to distinguish other types of tumors found within the retroperitoneum [7].
CD is a poorly understood condition that poses significant diagnostic and therapeutic challenges for physicians. The cause of CD is unknown, although several theories have been proposed to account for the spectrum of pathologic and clinical features. Chronic low grade inflammation [1], hamartomatous process [8], an immunodeficiency state, and autoimmunity [9], all are proposed as likely pathogenetic mechanisms. Epstein–Barr virus, Toxoplasma, and Mycobacterium tuberculosis are also implicated in some cases of CD [10].
Lymph nodes from the patients with CD implicate interleukin-6 as a causitive agent for the observed systemic manifestations [8].
In all types of CD, surgical resection is both recommended and essential for establishing a definitive diagnosis. With advancements in minimally invasive techniques, laparoscopic resection has increasingly been reported as a preferred approach in recent studies [9].
Keller et al. [11] conducted a retrospective review of 81 articles, comprising 74 cases of hyaline-vascular lesions, and 7 cases of plasma-cell lesions. Complete surgical excision proved to be curative for all patients in both groups.
Talat et al. [12] performed a systematic review and meta-analysis of 239 articles for a total of 404 patients with CD between 1954 and 2009. Of 278 patients with UCD, 249 patients underwent respective surgery, 13 had combined resective surgery with immunosuppressive therapy and 16 had immunosuppressive therapy alone. Ten years’ follow-up 5 revealed 13 disease related deaths. The authors found that in patients with UCD, surgical excision is safe and should be considered the gold standard for treatment.
Patients with UCD who underwent surgery (had significantly high overall survival (95.3%), 3 year disease-free survival (89.7%) and 5-year disease-free survival (81.2%). In patients who failed to be treated by resective surgery there has been reported mortality of 17.6% [12].
Regarding alternative treatment options for UCD, radiotherapy is the only well-documented approach. It can achieve clinical response and even cure in patients who are unsuitable for surgery or have undergone incomplete surgical excision. However, its use has been associated with potential complications and a high recurrence rate [13–15].
Conclusion
Given its rarity, CD is often overlooked in differential diagnoses, emphasizing the importance of a multidisciplinary approach for accurate diagnosis and optimal treatment planning. UCD typically has a favorable outcome with definitive surgical management, whereas MCD requires a multidisciplinary approach for effective treatment.
Conflict of interest statement
None declared.
Funding
No funding was received.
Consent for publication
Written informed consent was obtained from the patient for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.



