-
PDF
- Split View
-
Views
-
Cite
Cite
Yunfan Huang, Ya Wang, Chunming Sun, Xiaocheng Lu, Microsurgical treatment of spinal dural arteriovenous fistula with subarachnoid hemorrhage: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 2, February 2025, rjaf021, https://doi.org/10.1093/jscr/rjaf021
Close - Share Icon Share
Abstract
A rare spinal vascular malformation, the spinal dural arteriovenous fistula (sDAVF), is characterized by subtle clinical signs that make diagnosis and treatment of it highly challenging. In this case, we report a 70-year-old male who presented with subarachnoid hemorrhage, an unusual initial symptom of sDAVF. This report emphasizes the diagnostic complexities and highlights a successful management approach utilizing intraoperative indocyanine green angiography. Early identification and precise localization via advanced imaging techniques were pivotal in achieving a favorable outcome.
Introduction
The spinal dural arteriovenous fistula (sDAVF) is a rare vascular abnormality, which is distinguished by the irregularity of the connections between arteries and veins in the dura, potentially resulting in venous hypertension and ischemic alterations in the spinal cord. With an incidence of 5 to 10 cases per million annually, it accounts for 70%–80% of all spinal vascular malformations. Typically, middle-aged to elderly men are more affected [1]. With its typically insidious onset and non-specific symptoms, such as progressive myelopathy, early diagnosis of sDAVF is often challenging [2]. In this case, the patient presented with subarachnoid hemorrhage (SAH), a rare and atypical manifestation, making this an unusual presentation worth discussing. We provide details on the diagnostic and therapeutic strategies used in managing this case.
Case presentation
A 70-year-old male presented to the emergency department with sudden dizziness, severe headache, and persistent vomiting lasting 12 hours. He had no past history of major trauma, other diseases, or surgery. Initial head computed tomography (CT) revealed.
SAH predominantly in the posterior fossa, which was around the medulla oblongata, in the prepontine cistern and interpeduncular cistern (Fig. 1A). The following CT angiography (CTA) showed an abnormal vascular structure around the C1, but the image was not clear (Fig. 1B). Further digital subtraction angiography (DSA) revealed an arteriovenous fistula located at the C2 level, supplied by a branch of the left vertebral artery (Fig. 1C and D).
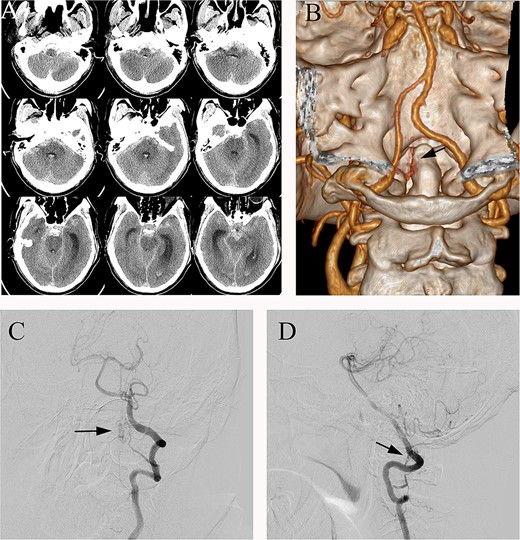
(A) Preoperative head CT, (B) CTA and (C, D) DSA identified the fistula (arrows) in the left lateral dural membrane and confirmed the intradural origin of the drainage vein.
The patient underwent microsurgical treatment of the sDAVF. A minimal midline incision was made between the foramen magnum and C2. Following dissection of the arachnoid membrane, the abnormal dilated venous structure was revealed. Intraoperative indocyanine green (ICG) videoangiography was performed to identify the proximal draining vein, which was filled early with the ICG (Fig. 2A). Then we coagulated the abnormal vein close to the dural (Fig. 2B). Then the arterialized veins were darked in ICG videoangiography (Fig. 2C), preserving the draining veins to avoid spinal cord ischemia. Postoperative DSA confirmed complete obliteration of the fistula, and the patient had an uneventful recovery (Fig. 3).
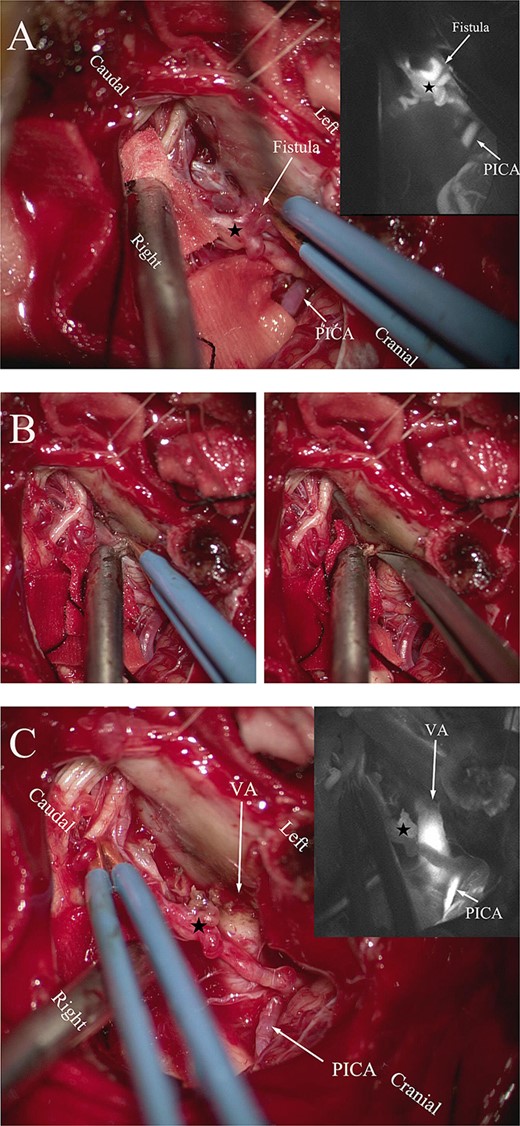
(A) Intraoperative ICG fluorescence imaging demonstrated that the fistula and abnormal drainage vein (asterisk) developed earlier than the posterior inferior cerebellar artery (PICA). (B) Coagulation of the origin of the abnormal drainage vein. (C) A subsequent ICG imaging confirmed the absence of fistula and drainage vein (asterisk) filling, with previously drainage veins developing later than the vertebral artery and PICA.
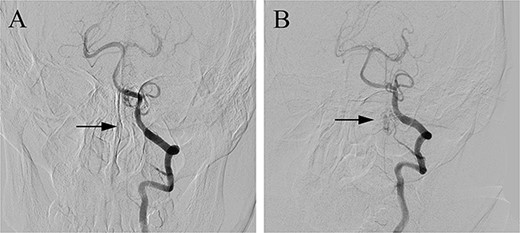
Postoperative DSA (A) confirmed the complete occlusion of the fistula (arrow) when compared with the preoperative DSA (B).
Discussion
Recent advancements in the treatment of sDAVF combined with SAH have significantly enhanced the management of this condition. Specifically, the literature consistently highlights microsurgery as the most commonly employed and effective treatment modality, particularly in cases involving complex anatomical configurations or multiple fistulas. By utilizing surgical ligation of the arteriovenous fistula, the risk of recurrence is effectively reduced, and long-term outcomes are improve [3, 4]. However, while interventional therapy offers a less invasive alternative in specific cases [5, 6], its higher recurrence rates compared to surgery make it less favorable for long-term management. As a result, microsurgical intervention is generally regarded as a more definitive and durable treatment option [4, 6]. In this context, the patient in our study underwent microsurgical treatment, with postoperative imaging confirming complete obliteration of the fistula.
Furthermore, accurate imaging plays a critical role in the diagnosis and management of sDAVF. For example, DSA remains the gold standard diagnostic tool, offering detailed visualization of the arterial supply and venous drainage associated with the fistula [3, 7]. Intraoperative fluorescence angiography using ICG has proven invaluable in guiding surgeons by precisely identifying abnormal venous outflow and confirming complete fistula closure during surgery [7]. Techniques such as magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) are also pivotal in preoperative planning [8], ensuring accurate localization and aiding in postoperative outcome evaluation. Thus, when these imaging modalities are combined, they not only enhance preoperative planning but also provide robust support for assessing surgical success.
Despite these advancements, the surgical treatment of sDAVF, especially when located at the craniocervical junction, presents significant challenges due to the complex anatomy of the spinal cord, nerve roots, and surrounding vasculature. Therefore, the choice of an appropriate surgical approach is crucial for the success of the operation. In our study, we employed the far-lateral transcondylar approach, which provided extensive exposure of the craniocervical region, facilitating safe manipulation around the C1 and C2 nerve roots while minimizing the risk of injury to critical nerves and blood vessels [4, 9].
With respect to outcomes, postoperative functional recovery varies among patients. As reported in the literature, successful sDAVF surgery typically results in marked improvement in motor and sensory functions [7]. In our study, 71.4% of patients achieved favorable functional recovery, with a modified Rankin Scale score of 0 to 1, indicating significant rehabilitation. However, certain complications, such as cerebrospinal fluid leakage and vocal cord paralysis, were observed [4, 7]. Nevertheless, these complications were effectively managed through prompt postoperative intervention. Furthermore, the literature emphasizes the importance of intraoperative neuromonitoring techniques, including somatosensory evoked potentials and motor evoked potentials, to mitigate the risk of neural injury during surgery [7].
Looking forward, despite the significant advancements in the surgical treatment of sDAVF, several areas for future development remain. First, the continuous evolution of imaging technologies, such as high-resolution MRI and 3D reconstruction, will enable more precise preoperative localization of the fistula [8, 10]. Second, with the increasing use of intraoperative fluorescence angiography, such as ICG, surgical precision will be further enhanced, reducing the incidence of residual fistulas and postoperative recurrence [7]. In addition, interventional therapy may offer a less invasive treatment option for high-risk patients [11, 12]. Finally, more long-term follow-up studies are needed to evaluate the efficacy of different treatment modalities and optimize clinical management strategies [8, 10].
Conflict of interest statement
None declared.
Funding
None declared.
References
Author notes
Chunming Sun and Xiaocheng Lu contributed equally to this work.



