-
PDF
- Split View
-
Views
-
Cite
Cite
Alban Déo Christian Opango, Zakaria Aziz, Abdelghafour Jaifi, Divina Ndelafei, Meriem El Fatihi, Nadia Mansouri Hattab, Pectoral major myocutaneous flap in our practice: about 25 cases, Journal of Surgical Case Reports, Volume 2024, Issue 7, July 2024, rjae448, https://doi.org/10.1093/jscr/rjae448
Close - Share Icon Share
Abstract
The pectoralis major myocutaneous flap (PMMF) was described by Ariyan in 1979 for head and neck reconstructions. It is a safe flap, currently supplanted by free flaps in developed countries, but which remains very useful in developing countries. We report a series of 25 cases of PMMF reconstruction. All patients were treated for advanced stages of oral cavity cancer, where tumor excision left significant tissue loss. The reconstruction used PMMF, taken using the same technique. Supplanted by free flaps in developed countries, PMMF remains useful in developing countries. It is a flap that has numerous advantages (ease of collection, viability, low requirements in terms of instrumentation, etc.). Many variations have been described over the years.
Introduction
First described by Ariyan in 1979, the pectoralis major myocutaneous flap (PMMF) has long been considered one of the main flaps for reconstructing defects of substance of the face and neck [1]. PMMF is nowadays increasingly neglected in the face of the rapid growth of free flaps, especially in developed countries. However, it remains of significant use in developing countries. The aim of this work is to show the place of PMMF in our practice.
We report a series of 25 patients treated at the stomatology and maxillofacial surgery department of the Mohammed VI University Hospital in Marrakech (Morocco) for maxillofacial tumors for which they underwent tumor excision with reconstruction by PMMF.
Series
The average age of our patients was 67 years. There was a male predominance with a sex ratio (M/F) of 2.1. The risk factors for oral cavity cancers mainly found were alcohol and tobacco intoxication in 21 cases and poor oral hygiene in 19 cases. The average consultation time was 9.5 months.
In all cases, it was a cancer of the oral cavity with a large tumor size classified as T4. The initial lesion was located at the level of the lip, the inner face of the cheek, the retromolar trigone or the floor of the mouth, with or without extension to the mandible. The histological diagnosis revealed squamous cell carcinoma in 22 cases (88%), adenoid cystic carcinoma in 2 cases (8%), and mucoepidermoid carcinoma in one case (4%). At the end of the extension assessment, the patients were classified stage IV (8th edition of the UICC).
All patients received, under general anesthesia, selective bilateral cervical lymph node dissection of areas I, II, and III; a tumor excision with minimum safety margins of 1 cm with or without mandibular resection leaving room for significant substance loss and a reconstruction using PMMF.
The flap was harvested with the patient in the supine position. The main landmarks (clavicle, acromio-xiphoid line, course of the pectoral branch of the acromio-thoracic artery, skin blade of the flap whose size depended on the loss of substance) were drawn. Then we made a cutaneous and subcutaneous incision up to the premuscular aponeurosis with the placement of stitches with an absorbable thread between the musculo-aponeurotic plane and the subcutaneous plane to avoid possible shearing. A large subcutaneous detachment of the anterior wall of the thorax around the skin blade was then carried out. We dissected between the pectoralis major and minor muscles at the level of the lateral edge of the pectoralis major muscle which was then released with an electrocautery from distal to proximal. Once the pedicle was located, the lateral and medial muscle cuts were continued, preserving a 2 cm muscular sleeve on either side of the pedicle. The pectoral nerve was severed. And the flap was then transferred to the recipient site through a fairly wide subcutaneous tunnel (in order to avoid any cervical stricture of the flap) allowing it to reach the lymph node dissection incision and then the recipient site. The flap was sutured, at the recipient site, with its skin blade intraorally. The muscular side was covered with fatty dressings and was subsequently subjected to skin grafting (Figs 1–6).
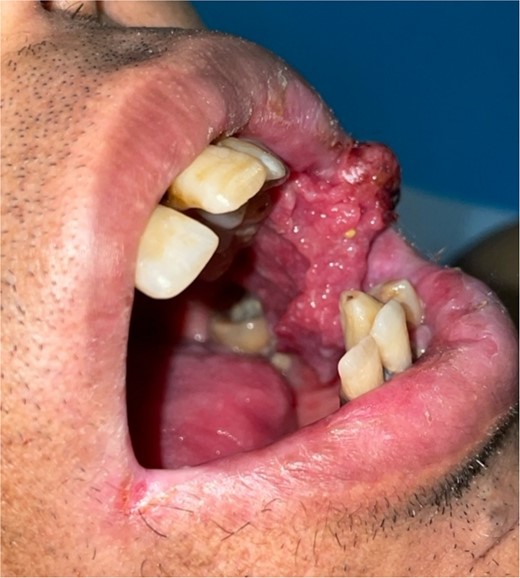
Patient with squamous cell carcinoma of the inner side of the left cheek.
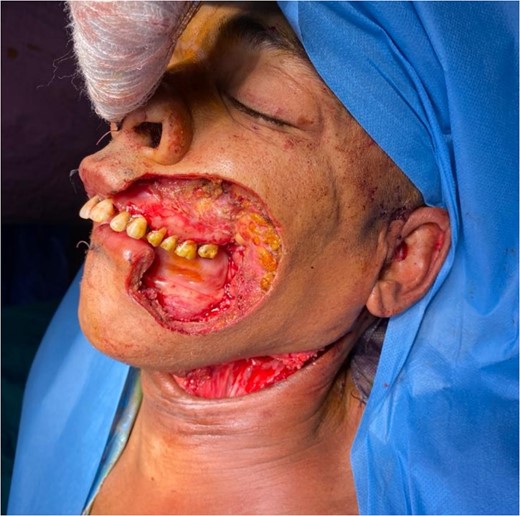
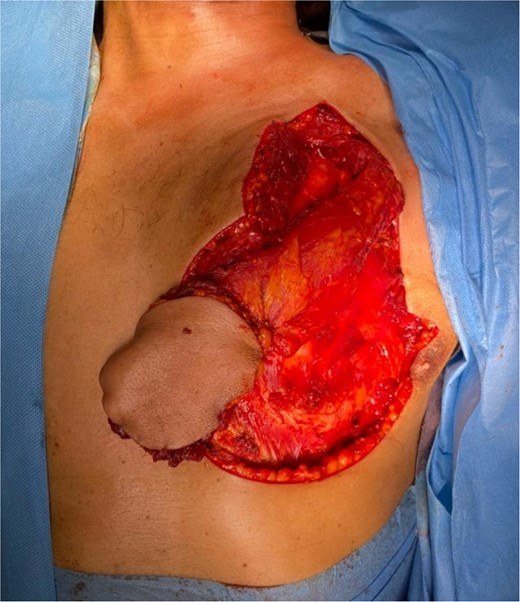
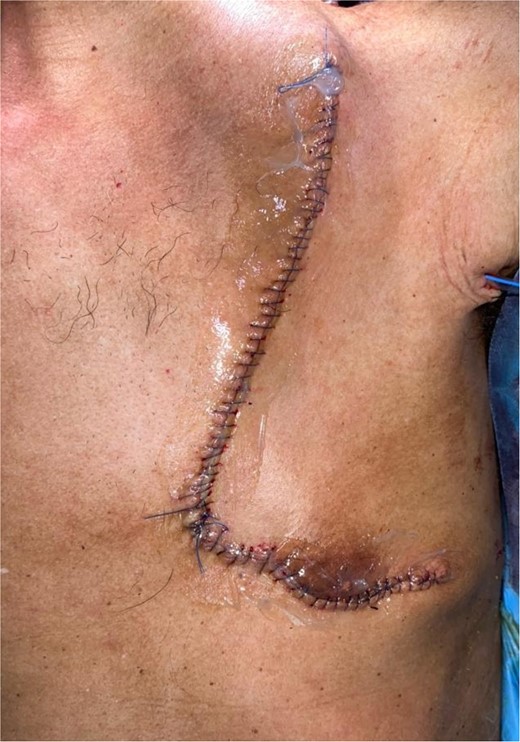
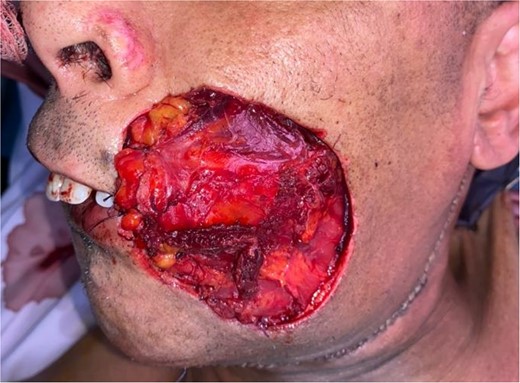
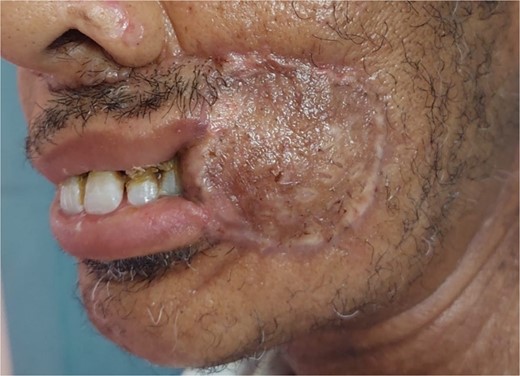
The development was generally favorable. We noted 1 case (4%) of total flap necrosis, 4 cases (16%) of partial flap necrosis, 2 cases (8%) of site infection managed by local care and appropriate antibiotic therapy. At the thoracic level, we had an inverted T-shaped scar in 60% of cases, and an L-shaped scar in 40% of cases. No complications were noted at the donor site.
Discussion
First described by Hueston and McConchie in 1968 as a rotating flap intended to repair a sternal defect, PMMF was redescribed by Ariyan in 1979 for head and neck reconstructions [2, 3]. PMMF was used as the workhorse for reconstruction of head and neck defects over the next three decades [4–7].
Nowadays, this flap is supplanted in developed countries by microanostomosed free flaps. Free flaps have an estimated risk of total necrosis of <5% and of partial necrosis of ~2% [8, 9]. Indeed, free flaps are considered the first choice in the majority of major head and neck defects due to their superior versatility, reliability, tissue compatibility, function, and esthetic results, as well as their low morbidity at the donor site. But successful free flap surgery requires a well-motivated and trained surgical team, good intraoperative flap monitoring, a well-equipped and readily available operating space, good laboratory support services, and availability of intensive care unit beds [10]. However, these conditions are not met in many developing countries, where the practice of microsurgery has remained largely rudimentary, or even non-existent. This justifies the use of safe flaps such as PMMF.
PMMF is a safe flap, with a rate of total necrosis estimated at 2% and partial necrosis estimated at 7–9% [11–13]. In addition to its safety, it has many advantages, including its proximity to the head and neck, the simplicity of harvesting and its use as an alternative in the event of failure of the microsurgical flap. It also provides a large quantity of skin and muscle, justifying its indications in the reconstruction of defects of substance of the neck (protection of the carotid), laryngopharynx, oropharynx, oral cavity, and anterior chest wall. However, the PMMF also has disadvantages, notably pain and limitations of neck movements, breast deformity especially in women, and chest scarring.
Over the years, the techniques for harvesting the PMMF have evolved greatly. There are currently several variations of skin palettes: elliptical, bilenticular, parallelograms, rhomboid, irregular, crescentric, sickle-shaped [11, 14]. The flap can be harvested without a skin blade; it is the pectoralis major myofascial flap. It can also be taken with a segment of rib, this is the osteomyocutaneous flap of the pectoralis major [15]. Modifications have also been made in order to obtain an increase in its arc of rotation [16, 17].
PMMF is a flap that remains very useful in the reconstruction of facial and neck defects in developing countries where the technical support remains limited and where access to microsurgery is limited.
Conflict of interest statement
None declared.
Funding
None declared.



