-
PDF
- Split View
-
Views
-
Cite
Cite
Phoebe C Wood, Fraser H Simpson, Katie Lehane, Peter Yuide, Robert Franz, Atraumatic splenic rupture as first presentation of hairy cell leukaemia—a case report, Journal of Surgical Case Reports, Volume 2024, Issue 7, July 2024, rjae437, https://doi.org/10.1093/jscr/rjae437
Close - Share Icon Share
Abstract
Atraumatic splenic rupture is a complex surgical pathology owing to its rarity, non-specificity of symptoms and gravity of possible outcomes. This case outlines the investigation and management of a patient with atraumatic splenic rupture secondary to undiagnosed hairy cell leukaemia. While the patient was initially managed conservatively, they went on to have a splenectomy owing to ongoing transfusion requirements. A review of the literature has also been performed and presented to highlight the potential causes of atraumatic splenic rupture and the various options for confirming diagnosis and definitive management.
Introduction
Splenic rupture most commonly occurs in the setting of abdominal trauma and can become life-threatening. Rarely, splenic rupture is not a result of trauma but of underlying pathology, be it infectious, malignant, metabolic, or vascular. This is termed atraumatic splenic rupture (ASR). Unfortunately, ASR can go unrecognized and untreated owing to its rarity and occult nature of underlying pathologies at time of presentation [1]. Therefore, ASR requires a high level of clinical suspicion [2]. This report outlines the case of a 46-year-old female presenting to the emergency department with haemoperitoneum secondary to ASR of unknown aetiology. They underwent a splenectomy and were diagnosed with Hairy Cell Leukaemia (HCL). This report outlines important diagnostic and management considerations in ASR.
Case report
A 46-year-old female presented to the emergency department with 2 days of vomiting, shoulder pain, dyspnoea, and presyncope. The patient denied abdominal trauma or relevant medical history.
At triage, the patient had a respiratory rate of 28, oxygen saturation of 88% on room air, sinus tachycardia at 130 bpm, systolic blood pressure of 110 mmHg, and cool peripheries. She was GCS 15 and afebrile. She was pale and dyspnoeic. Her abdomen was moderately distended with localized peritonism over the left upper quadrant. There were multiple ecchymoses on both legs.
Initial blood tests revealed a haemoglobin of 7.6 g/dL, white cell count of 12 400/μL, platelet count 106 × 109/L, pH of 7.22, and lactate of 10 mmol/L. A focused assessment with sonography for trauma (FAST) was performed showing free fluid in all four quadrants of the abdomen.
After resuscitation, the patient’s haemodynamic profile improved, and she then had a CT of the abdomen and pelvis with multiphase contrast to assess for active bleeding.
Imaging showed a subphrenic haematoma measuring 15 × 12 cm within the left upper quadrant and moderate volume haemoperitoneum. No active haemorrhage was detected. There were three hypodense splenic lesions of unknown aetiology (vascular, haematologic, or metastatic) and mild splenomegaly (Fig. 1). A diagnosis of atraumatic splenic rupture was made.
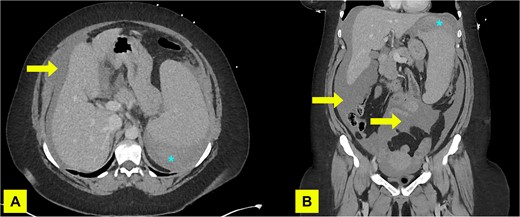
A contrast enhanced CT of abdomen and pelvis (portal venous phase) with axial (A) and coronal (B) views performed on admission demonstrating haemoperitoneum (arrows) and subcapsular spleen haematoma (asterisk).
Blood films from admission revealed numerous intermediate-large lymphocytes with voluminous cytoplasm and ruffled membranes with bean shaped/reniform nuclei, concerning for circulating lymphoma cells. Haematology was consulted, and a diagnosis of HCL was made based on the blood film and flow cytometry. Platelets and red blood cells were transfused as required.
Over 48 hours, the patient remained transfusion dependent. Interventional radiology opinion was sought; however, it was felt that the bleed would not be amenable to embolization. The decision was made to proceed to open splenectomy. The operation was uncomplicated, and 3.5 L of blood and clot were found intra-abdominally. The spleen was sent for histopathology.
Macroscopically, the spleen weighed 1089 g and measured 200 × 150 × 80 mm with rupture at the superior pole and stripping of the capsule with underlying haematoma. Microscopically, there was intraparenchymal haemorrhage with extensive involvement of red pulp and partial effacement of the hilar lymph nodes by infiltrate cells consistent with HCL (Fig. 2A–C). These lesional cells show strong diffuse expression of CD20 and were negative for CD5, CD10, and CD23. Flow cytometry performed on this specimen demonstrated an abnormal population of cells consistent with a mature B-cell lymphoproliferative disorder with an expression profile in keeping with HCL.
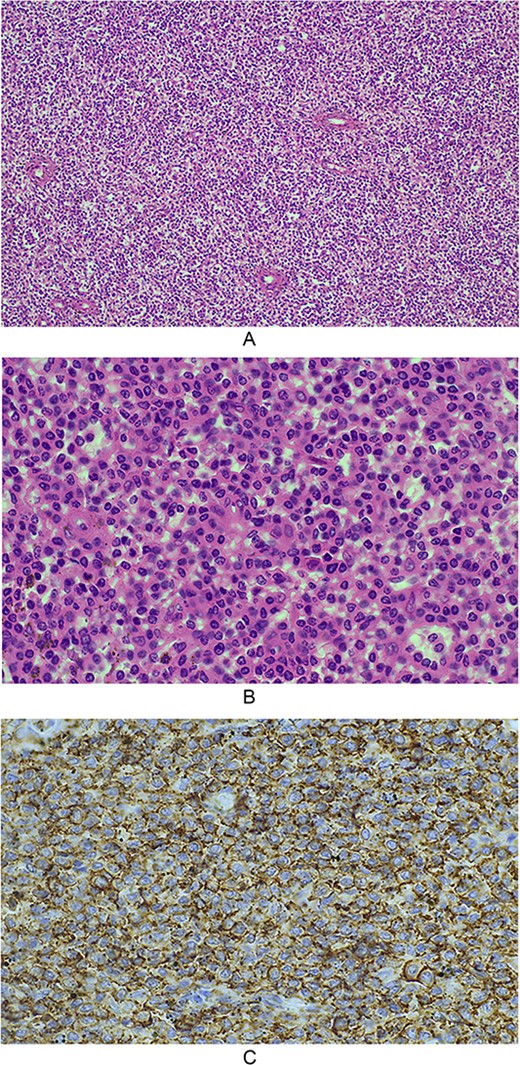
(A) 10× magnification, splenic architecture is effaced by a diffuse infiltrate of homogenous cells, which are expanding the red pulp cords and involving sinuses, with minimal white pulp seen; (B) 40× magnification, the infiltrative cells have ovoid to reniform nuclei with abundant pale eosinophilic cytoplasm. Mitotic activity is not readily identified; (C) microscopic photograph at 40× magnification demonstrates the lesional cells showing strong diffuse positive expression of CD20.
The patient was admitted to ICU and was stepped down to the ward at Day 1 postoperatively. Postoperative course was uncomplicated. The patient was started on prophylactic antibiotics and referred to haematology as an outpatient. She has since been commenced on methylprednisolone and cladribine.
Discussion
ASR is defined as splenic rupture in the absence of significant abdominal trauma [1]. While commonly pathological, where rupture is precipitated by underlying disease, it can be spontaneous, with no cause found [1]. Precipitants can be infectious, rheumatological, anatomical, pharmacological, haematological, and malignant, with haematological malignancy being most common [1, 2]. Splenic rupture may be the first sign of disease [3]. Patients presenting with ASR should be investigated for underlying pathology. Spontaneous rupture should be a diagnosis of exclusion.
Rupture may be acute or delayed [1]. Male sex, adulthood, severe splenomegaly, and cytoreductive chemotherapy are risk factors for rupture in haematological malignancy [4].
Infiltration and dissolution of the splenic capsule by leukaemia cells is thought to be the underlying mechanism for ASR [1, 2]. Intrasplenic tension, infarction, and disruptions in coagulation may also contribute [2, 5]. Splenomegaly has a controversial role in ASR. Theoretically, the lowering of the spleen edge below the ribs exposes friable tissue to trauma; however, rupture can occur in the absence of splenomegaly [2, 5].
Clinical presentation of ASR depends on the volume of bleeding and underlying pathology. Patients may report pain to the left upper quadrant of the abdomen. Diaphragmatic irritation can lead to shoulder pain. Commonly, patients present with anaemia and hypovolaemic shock if left untreated [2]. Patients with HCL may have more significant bleeding as they often present with thrombocytopaenia [5, 6].
Traditionally, paracentesis was used to confirm the presence of blood in the peritoneal cavity when suspicion for ASR was high [2]. More recently, FAST and CT scanning is more commonly employed, with the former being a valuable tool to assess for intraperitoneal fluid in unstable patients and the later providing information on the nature and source of said fluid. CT may also determine the grade of rupture [2].
Management of splenic rupture can be conservative, radiological, or surgical [2]. Surgical management with splenectomy is most common [7]. Appropriate haemoderivative support is also vital [2]. Surgical management is particularly important when there is suspicion or confirmation of underlying haematological malignancy [1]. However, there are cases of splenic rupture in HCL that have been treated with embolisation only [6].
Mortality of ASR is estimated between 12.2% and 20% [8]. Poor prognostic factors include splenomegaly, age over 40, neoplastic disorders, and delayed diagnosis and management [8].
Conclusion
Splenic rupture should be considered a differential diagnosis for patients with haemodynamic instability and abdominal pain even in the absence of trauma. ASR should also prompt investigation into underlying pathology that may require further treatment. This is one of only a few cases of atraumatic splenic rupture as a first presentation of hairy-cell leukaemia. It highlights the importance of considering ASR in the atypical patient and showcases both conservative and surgical management.
Conflict of interest statement
None declared.
Funding
None declared.



