-
PDF
- Split View
-
Views
-
Cite
Cite
Lina Rodríguez, Martha Monroy, Marco E Guerrero, Natalia Villarroya, Von Willebrand’s disease in breast surgery: case report, Journal of Surgical Case Reports, Volume 2024, Issue 6, June 2024, rjae395, https://doi.org/10.1093/jscr/rjae395
Close - Share Icon Share
Abstract
Von Willebrand disease is an inherited disorder characterized by deficiency of von Willebrand factor, which contributes to platelet adhesion to the endothelium. Patients with coagulation disorders present a challenge at the time of surgery due to the high risk of presenting heavy bleeding within the procedure or postoperative hematomas. We present a case of a 56-year-old woman with Type 1 von Willebrand’s disease who was scheduled for breast explantation with autologous reconstruction, due to the presence of long-standing breast implants. The case was satisfactorily managed by a multidisciplinary team formed by plastic surgery, hematology, and anesthesiology, individualizing the management for the patient’s case, obtaining good results and a safe procedure.
Introduction
Erick von Willebrand described in 1925 the disease that would later bear his name.
Von Willebrand’s disease (VWD) is a disorder of von Willebrand factor (VWF), which is a glycoprotein that contributes to platelet adhesion to the endothelium and is a carrier protein for plasma factor VIII [1].
It is the most common hereditary coagulation disorder described, occurring in 1%–2% of the general population and predisposing to mucocutaneous, posttraumatic, or postoperative bleeding. Depending on its severity and the functionality of the VWF present in the patient, different complications may occur during surgical procedures [2]. Unlike other disorders such as hemophilia, the incidence in men and women is very similar; however, women are diagnosed more frequently due to the alterations in the menstrual cycle in which studies are carried out and in which this disorder is found incidentally [3].
This pathology is differentiated into three types according to deficiencies and associated cofactors. Type 1 is characterized by a partial deficiency of VWF, type 2 also presents a partial deficit and is defined by qualitative variants in different subtypes (A, B, N, M) according to cofactor alterations, while type 3 is the total absence of VWF [4] (Table 1).
Types of von Willebrand disease, VWF: Ag = von Willebrand factor antigen, VWF: Rco = von Willebrand factor ristocetin receptor activity, VWFIII: factor VIII N: normal
| . | Von Willebrand Disease Clasification . | ||||||
|---|---|---|---|---|---|---|---|
| Normal . | Type 1 . | Type 2A . | Type 2B . | Type 2 N . | Type 2 M . | Type 3 . | |
| FvW: Ag | N | ↓ | ↓ | ↓ | N o ↓ | o N↓ | Absent |
| FvW: Rco | N | ↓ | ↓↓↓ | ↓↓ | N o↓ | ↓↓ | Absent |
| F VIII | N | o N↓ | N o↓ | N o↓ | ↓↓ | N | 1–6% |
| . | Von Willebrand Disease Clasification . | ||||||
|---|---|---|---|---|---|---|---|
| Normal . | Type 1 . | Type 2A . | Type 2B . | Type 2 N . | Type 2 M . | Type 3 . | |
| FvW: Ag | N | ↓ | ↓ | ↓ | N o ↓ | o N↓ | Absent |
| FvW: Rco | N | ↓ | ↓↓↓ | ↓↓ | N o↓ | ↓↓ | Absent |
| F VIII | N | o N↓ | N o↓ | N o↓ | ↓↓ | N | 1–6% |
Types of von Willebrand disease, VWF: Ag = von Willebrand factor antigen, VWF: Rco = von Willebrand factor ristocetin receptor activity, VWFIII: factor VIII N: normal
| . | Von Willebrand Disease Clasification . | ||||||
|---|---|---|---|---|---|---|---|
| Normal . | Type 1 . | Type 2A . | Type 2B . | Type 2 N . | Type 2 M . | Type 3 . | |
| FvW: Ag | N | ↓ | ↓ | ↓ | N o ↓ | o N↓ | Absent |
| FvW: Rco | N | ↓ | ↓↓↓ | ↓↓ | N o↓ | ↓↓ | Absent |
| F VIII | N | o N↓ | N o↓ | N o↓ | ↓↓ | N | 1–6% |
| . | Von Willebrand Disease Clasification . | ||||||
|---|---|---|---|---|---|---|---|
| Normal . | Type 1 . | Type 2A . | Type 2B . | Type 2 N . | Type 2 M . | Type 3 . | |
| FvW: Ag | N | ↓ | ↓ | ↓ | N o ↓ | o N↓ | Absent |
| FvW: Rco | N | ↓ | ↓↓↓ | ↓↓ | N o↓ | ↓↓ | Absent |
| F VIII | N | o N↓ | N o↓ | N o↓ | ↓↓ | N | 1–6% |
Replacement of VWF by concentrates is currently recommended, while the dose and duration of treatment depend on laboratory tests and should be closely monitored by laboratory assessment of ristocetin cofactor activity of (VWF: RCo). Adjunctive antifibrinolytic agents, such as tranexamic acid, are useful tools to control mucocutaneous bleeding and have been widely used in surgery [5].
Patients with coagulation disorders present a challenge at the time of surgery due to the high risk of heavy bleeding within the procedure or the risk of presenting with postoperative hematomas. In this article, we report the case of a female patient with VWD type 1 who underwent multidisciplinary management with favorable results and without complications.
Case report
A 56 years old female patient comes for breast explantation due to the presence of implants for 16 years. Within the relevant clinical history, she presents Von Willebrand disease diagnosed 10 years ago, hypermenorrhea, hypercholesterolemia in management with rosuvastatin, no previous transfusions, augmentation mammoplasty during which she experienced abundant bleeding, no consumption of cigarettes or alcohol, two pregnancies, two births, and a family history of VWD with sister. Given the history, multidisciplinary management with anesthesiology and hematology is initiated.
Physical examination revealed a patient weighing 67 kg, in good general condition, with bilateral breast augmentation + ptosis (Fig. 1), Mallampati I. Preoperative laboratory studies showed hemoglobin 13.2 g/dl, hematocrit 39.9%, platelets 283 000 μl, VW Ag 36 μ/l, factor VIII 16 μ/l, ratio < 0. 7, ristocetin <10, minimally decreased multimers, VWF activity 22.3%, VWF 33.9%, factor VIII 30%, normal platelet aggregation curve, no prolongation of clotting times, glucose 91 mg/dl, creatinine 0.8 mg/dl.
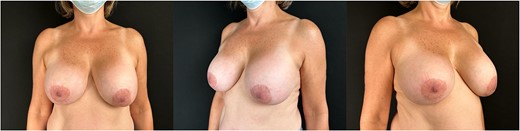
Patient 56 years old with breast implants of 16 years with bilateral breast ptosis; patient is classified with a type I VWD, non-bleeding genotype, due to an antigen/cofactor ratio > 0.6.
For surgical intervention, it is necessary to reach factor VIII activity >80% and maintain 50% for the first 3 postoperative days, so it is indicated:
Administer Factor VIII /VWF VIII 1500 IU IV, 30 min before surgery.
Administer Factor VIII /VWF VIII 1500 IU IV 12 h after surgery.
Administer Factor VIII/Von Willebrand’s Factor 1000 IU IV every 12 h on Days 2, 3 and 4 postoperatively.
On Day 5, according to the clinical evolution, 1000 IU of Factor VIII/Von Willebrand will be administered.
The patient received a substitution scheme with a body weight-adapted dose of recombinant factor under close laboratory surveillance to maintain optimal levels. This scheme, sufficient proactive hemostasis during surgery, early postoperative mobilization and the use of compression garments are necessary measures to avoid adverse events.
Anesthesiology calculated a maximum bleeding of 1305 cc to reach a minimum hemoglobin of 9 mg/dl (permissible blood loss), before the procedure, according to her baseline parameters. A reserve of two units of red blood cells was made. One gram of tranexamic acid was administered 30 min before starting the procedure. Basic monitoring was performed for the procedure (non-invasive blood pressure control, electrocardiography in DII/V5 derivation, pulse oximetry, and capnography). A balanced general anesthesia (inhaled/intravenous) with standard orotracheal intubation was used for induction: 100 μg of fentanyl, 150 mg of propofol, 80 mg of lidocaine 2% without epinephrine, and 30 mg of rocuronium. For anesthesia maintenance, remifentanil was administered at a rate of 0.2 μg /kg/min, and sevoflurane was maintained at a concentration of 1 volume % at low flow. As antithrombotic measures, we used permanent antithrombotic stockings, pneumatic intermittent compression stockings together with a 10° flexion of the knees was maintained during the transoperative period, temperature control was achieved with local heat with thermal sheet and hot liquids to maintain temperatures of 32°C. Postoperative vomiting prophylaxis and analgesia were performed, pain pump was not used to avoid more venipuncture points, and pain management was performed during her hospitalization.
The patient followed the indicated scheme, achieving the proposed goals of factor VIII activity and underwent surgery.
Plastic surgery performs surgical procedure under Wise pattern marking (Fig. 2), with supero-medial pedicle technique for left areola and superior pedicle for right areola, bilateral explantation and capsulectomy + hybrid reconstruction only in the left breast (lipoinjection of 30 cc of fat for symmetry), and with autologous tissue with inverted T closure. Proactive hemostasis was performed intraoperatively. Bilateral drains were left. Patient did not present complications, duration of the procedure 180 min, with almost none bleeding.
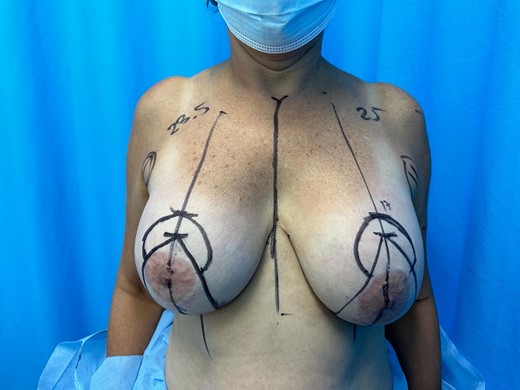
Preoperative marking detailing the distance of the right nipple areola complex: 23.5 cm left 25 cm and point A marked at 17 cm bilaterally under wise pattern.
She was successfully extubated, and the use of a permanent postsurgical bra, avoiding sleeping face down, and early assisted ambulation were indicated. The patient remained hospitalized 72 h for the management established by hematology and was monitored after hospital discharge at 24 h, 3 days and 7 days postoperatively, at which point drains were removed when presenting a deficit <30 ml in 24 hours. Subsequently monthly controls were conducted without presenting neither surgical nor clinical complications inherent to the procedure or to his medical history, laboratory values remained within normal ranges (Fig. 3).
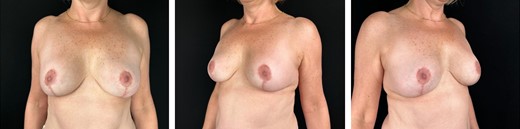
Postoperative 6-month breast explantation + autologous reconstruction without complications; left breast with hybrid technique.
Discussion
The risk of presenting complications in breast surgery is ~12% in general population, the most frequent within this group being hematoma, infection, dehiscence, and seroma [6]. In breast surgery, it is reported that hematoma formation occurs in ~5.1% of the operated patients of which 1.1% will require blood transfusion [7].
Patients with coagulation disorders such as VWD have a higher risk of intraoperative and postoperative bleeding, so the risk of hematoma formation is potentially higher [8]. Taking this into account, it is very important to perform an adequate and meticulous hemostasis during surgery. It is not recommended to perform blunt or blind dissections in the repositioning of autologous reconstruction tissue, since this can generate preventable complications such as hemodynamic alterations, decompensation, or the requirement of blood transfusions in case of abundant bleeding.
The hematoma is clinically recognizable and very characteristic. It presents as an edematous breast, generating a marked asymmetry with the contralateral breast. It is also a painful breast to palpation and movement of the ipsilateral arm [9].
The perioperative management of patients with VWD depends on its type and severity, the most frequent type is type 1 (70%–80%) of the cases [10]. The main objective of the approach to patients is to correct the VWF deficiency by means of VWF replacement, individualizing the management according to the deficiency presented by the patient. It should be considered that products containing F VIII + VWF in high doses can generate thrombotic risks by generating much higher levels of F VIII, so in case of a very severe deficiency it is recommended to use products containing only VWF, which intrinsically increases the levels of F VIII in a safer way [11].
To reduce the risks, additional measures are used, such as the use of tranexamic acid, which is a synthetic lysine that inhibits fibrinolysis by blocking the plasminogen coupling sites. Tranexamic acid remains ~8 h in the blood and up to 17 h in the tissues after its application [12]. It has been described that it can reduce the incidence of hematoma and a minor decrease in postoperative hemoglobin by placing it 30 min before surgery in doses of 1 g intravenously, particularly after aesthetic procedures [13]. The use of pneumatic and mechanical compression stockings was added to the management. Early postoperative assisted mobilization and multidisciplinary support including hematology and anesthesiology create a safe environment for elective procedures in patients with VWD.
Conclusions
This case report evidences the importance of individualized multidisciplinary management in patients who are going to undergo plastic surgery and present this coagulation disorder. All the measures applied in the case resulted in a favorable procedure, keeping the parameters within normal ranges. A combination of good surgical technique and close anesthetic monitoring allow us to perform safe procedures.
Conflict of interest statement
None declared.
Funding
None declared.



