-
PDF
- Split View
-
Views
-
Cite
Cite
Takaaki Suwa, Nobutaka Kawamoto, Shunsuke Morita, Hiroshi Hasegawa, Junichi Zaitsu, Keizo Misumi, Pulmonary tuberculoma-induced cyst formation leading to repeated pneumothorax: a case report, Journal of Surgical Case Reports, Volume 2024, Issue 5, May 2024, rjae365, https://doi.org/10.1093/jscr/rjae365
Close - Share Icon Share
Abstract
Most cases of secondary spontaneous pneumothorax in patients with active pulmonary tuberculosis are caused by rupturing of the visceral pleura caused by Mycobacterium tuberculosis. The check-valve airway mechanism in the lungs is generally involved in the formation of pulmonary cysts, which often cause spontaneous pneumothorax. Herein, we describe a rare case of repeated spontaneous pneumothorax suspected to have been caused by pulmonary cyst formation as a result of a tuberculoma. The patient was a man with a family history of pulmonary tuberculosis. Pulmonary cysts were gradually enlarged on the peripheral side of a lung mass in the upper lobe of the patient’s right lung, who experienced two spontaneous pneumothoraxes in the area. Exploratory surgery was performed to diagnose the lung mass and treat the pneumothorax, resulting in a final diagnosis of pulmonary tuberculoma. A check-valve mechanism caused by the pulmonary tuberculoma was suspected based on the patient’s clinical course.
Introduction
Spontaneous pneumothorax can be classified into primary and secondary types [1]. Secondary spontaneous pneumothorax is considered a complication of an underlying pulmonary disease such as chronic obstructive pulmonary disease, cystic lung disease, malignancy, pulmonary infections, or interstitial lung diseases [2]. Secondary spontaneous pneumothorax develops in 1%–2% of patients with active pulmonary tuberculosis [3], and most cases are caused by rupture of the visceral pleura as a result of Mycobacterium tuberculosis infection [4].
The check-valve mechanism of the airway is key to the formation of pulmonary cysts, which in turn lead to spontaneous pneumothorax, wherein air becomes progressively trapped in alveoli that are distal to the obstruction [5]. Most cases of pulmonary cyst formation associated with lung masses are later diagnosed as cases of lung cancer [6]. In rare cases, obstructive bronchiolitis caused by an infectious pulmonary disease can give rise to a check-valve phenomenon that results in the formation of pulmonary cysts on the peripheral side of the affected lung [7].
Herein, we report a surgical case of repeated secondary spontaneous pneumothorax, wherein pulmonary cysts were considered to have formed through a check-valve mechanism caused by pulmonary tuberculoma. To our knowledge, this is the first report of pulmonary tuberculoma resulting in pulmonary cyst formation.
Case report
The patient, a 75-year-old man, had a history of type 2 diabetes mellitus, dyslipidaemia, hypertension, hyperuricaemia, and alcohol-related chronic pancreatitis and was obese (body mass index, 35.1 kg/m2). He had quit smoking at 60 years of age, with a 60-pack-year smoking history. He had a family history of pulmonary tuberculosis on his father’s side.
During the computed tomography (CT) scan of the 62-year-old patient, physicians did not point out infiltrating shadows in the right upper lobe caused by suspected pulmonary tuberculosis. Therefore, a chest CT scan was not performed until the patient developed a right pneumothorax at age 70. At 70 years old, the patient developed pneumothorax in his right lung; a CT scan revealed a 3.2 cm mass with satellite lesions in the right upper lobe, as well as pulmonary cysts on the peripheral side of the mass (Fig. 1). At this point, a retrospective review of the patient’s earlier CT images (at age 62 years) confirmed that there were some shadows indicative of pulmonary infiltration in the upper lobe of the right lung, although pulmonary cysts were not visible. The pulmonary cysts were considered to have caused the patient’s pneumothorax, which resolved following chest drainage. A bronchoscopy was performed after the pneumothorax improved, with brushing cytology and bronchoalveolar lavage cytology revealing no malignant cells. An acid-fast bacilli culture of the patient’s bronchoalveolar lavage fluid and the T-cell spot test for tuberculosis infection (T-SPOT.TB) were both negative. An 18F-fluorodeoxyglucose positron emission tomography/CT scan showed a maximum standard uptake value (SUVmax) of 1.8 in the lung mass. Since the lung mass was not suspected to be aggressively malignant [8], careful follow-up was performed, involving another CT scan. The lung mass was not enlarged; however, some growth of the pulmonary cysts was observed (Fig. 2).
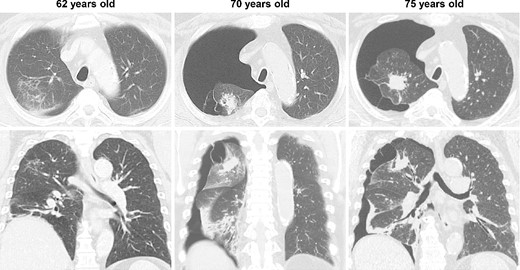
CT imaging findings; at age 62, shadows suggesting pulmonary infiltration were present in the upper lobe of the patient’s right lung, but no pulmonary cysts were observed; at age 70, the patient developed spontaneous pneumothorax in his right lung; another scan revealed a 3.2 cm lung mass with satellite lesions in the upper lobe of the right lung, as well as pulmonary cysts on the peripheral side of the mass; at age 75, the patient experienced a relapse of the spontaneous pneumothorax in the same lung.
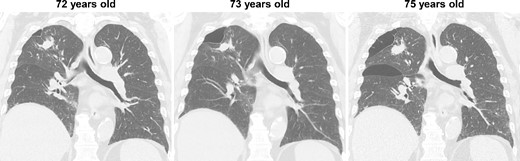
CT images of the intervening period between the initial and relapsed pneumothorax; no enlargement of the lung mass was observed, but gradual enlargement of the pulmonary cysts was apparent.
At 75 years old, the patient’s right spontaneous pneumothorax relapsed (Fig. 1). Exploratory surgery was performed to diagnose the lung mass and treat the pneumothorax. The lung mass and pulmonary cysts were excised (Fig. 3A and B). The lung mass was diagnosed as a granuloma with necrosis through analysis of frozen surgical biopsy sections, and a polymerase chain reaction (PCR) test for tuberculosis was positive. The operative time was 134 min, and the estimated blood loss was 15 ml. No postoperative air leakage was observed, and the patient’s chest drain tube was removed on postoperative Day 2. He was discharged on postoperative Day 12, following respiratory rehabilitation due to his obesity. The timeline of the patient’s clinical course is presented in Fig. 4.
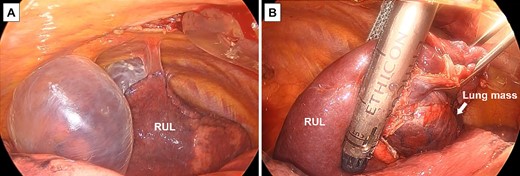
Surgical findings; (A) pulmonary cysts were observed in the upper lobe of the patient’s right lung; (B) the lung mass and pulmonary cysts were removed using a surgical stapler; RUL, right upper lobe.
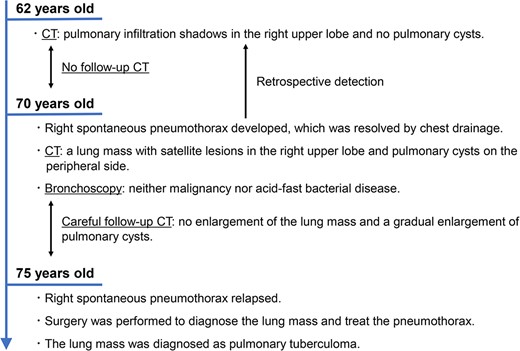
Timeline of the patient’s clinical course; CT, computed tomography.
Macroscopic findings revealed bronchioles surrounded by a pale-yellow lung mass, with pulmonary cysts on the peripheral side of the mass (Fig. 5A). Histopathological findings indicated that the mass was a caseous granuloma with multinucleated Langhans giant cells and lymphocyte accumulation (Fig. 5B). This was diagnosed as a pulmonary tuberculoma, based on the PCR results. Lymphocyte infiltration and interstitial tissue fibrosis associated with chronic inflammation were observed around the bronchioles (Fig. 5C). Neither bronchial obstruction nor evidence of malignancy was observed.
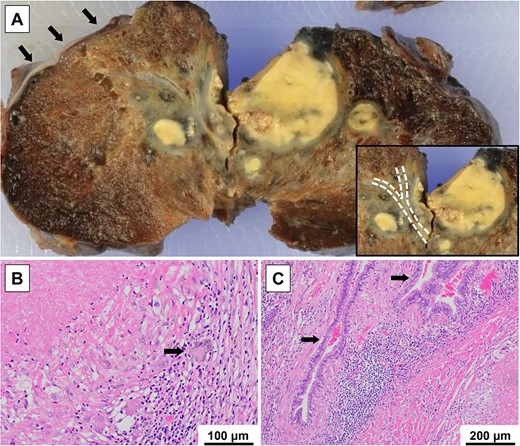
Macroscopic and histopathological findings; (A) a pale-yellow lung mass surrounding the bronchioles (dotted lines) and pulmonary cysts (arrows) on the peripheral side of the mass were observed; (B) a caseous granuloma with multinucleated Langhans giant cells (arrow) and accumulation of lymphocytes was observed; this was diagnosed as a pulmonary tuberculoma, after considering the result of a PCR test; (C) lymphocyte infiltration and fibrosis of the interstitial tissue associated with chronic inflammation were observed around the bronchioles (arrows); neither bronchial obstruction caused by the lung mass nor emphysematous changes in the background lung were observed.
One month postoperatively, the patient was started on isoniazid, rifampicin, and ethambutol. No pneumothorax recurrence was observed at a 9-month postoperative follow-up.
Discussion
Tuberculosis is a global health concern. Although the number of patients with tuberculosis in Japan is decreasing, 8.2 persons per 100 000 population are expected to develop it in 2022 [9]. T-SPOT.TB is used to detect latent tuberculosis infection, with a sensitivity of 81%–88% [10, 11]. Therefore, some false-negative results do occur. Pulmonary tuberculoma occurs in ~9% of patients with pulmonary tuberculosis [12]. The CT findings of pulmonary tuberculoma often show a lung mass with satellite lesions [13]. However, differentiating pulmonary tuberculoma from lung cancer via clinical and imaging findings is challenging [14]. Pulmonary tuberculoma should be considered whenever lung masses with satellite lesions are observed, even if bronchoscopy or T-SPOT.TB results are negative.
Most cases of pulmonary cyst formation associated with lung masses result from lung cancer [6], with infectious pulmonary diseases representing an exceedingly rare cause [7]. In our case, there were initially no pulmonary cysts in the upper lobe of the patient’s right lung. When a pulmonary tuberculoma was identified via CT, pulmonary cysts on the peripheral side of the mass were also observed for the first time. These cysts then enlarged over time, suggesting that they were caused by a check-valve mechanism in the bronchioles that were surrounded by the pulmonary tuberculoma. Surgical treatment to prevent pneumothorax and diagnose the lung mass may be advisable in cases of enlarged pulmonary cysts associated with lung masses.
A major limitation of this case is the difficulty of conclusively demonstrating the suspected check-valve mechanism behind the patient’s cysts, based on histopathological and CT findings alone. Reviews of similar case reports may help to clarify this hypothesis.
Acknowledgements
We would like to thank Editage (http://www.editage.jp) for their assistance with English language editing.
Conflict of interest statement
None.
Funding
None.



