-
PDF
- Split View
-
Views
-
Cite
Cite
Luz Estefanía González Gallegos, Carlos Alberto Córdova Velázquez, Oscar Chapa Azuela, Irving Hugo Aguilar Preciado, Carmen Judith Roca Vásquez, Jorge Alberto Roldan García, Chylous ascites after associating liver partition and portal vein ligation for stage hepatectomy (ALPPS): overview and case report, Journal of Surgical Case Reports, Volume 2024, Issue 5, May 2024, rjae357, https://doi.org/10.1093/jscr/rjae357
Close - Share Icon Share
Abstract
Chylous ascites is an uncommon pathology with low incidence following hepato-pancreato-biliary surgery, there are no cases reported in the international literature following the associating liver partition and portal vein ligation for stage hepatectomy (ALPPS) procedure. It is caused by abnormal intraperitoneal accumulation of lymph fluid in the abdominal cavity secondary to obstruction or injury to the chyle cistern or its tributaries. We describe the case of a 49-year-old woman diagnosed with colon cancer and liver metastasis. ALPPS was performed, on a first and second stage, presenting a high drainage output as well as change in the characteristics of the drainage fluid. The diagnosis of chylous ascites was confirmed by finding triglyceride levels in the drainage fluid at 300 mg/dL. Medical treatment was started based on a hyper-protein diet and fat restriction, supplemented with medium-chain triglycerides and somatostatin analog, with fistula resolution. It can be managed with medical treatment.
Introduction
Postoperative (PO) chylous ascites is an uncommon complication after liver surgery, with only a few cases reported. To our knowledge, there are no cases reported in the literature following associating liver partition and portal vein ligation for stage hepatectomy (ALPPS) procedure. This entity can generate a state of malnutrition and immunodeficiency given the composition of the chyle consisting of long-chain triglycerides, lymphocytes and immunoglobulins; in the context of major liver surgery, it could represent an increase in morbidity and mortality. Currently, there is no standard treatment; usually, it is determined by the clinical condition of the patient and institutional experience [1]. We describe the case of a patient who developed chylous ascites in the PO course after an extended right hepatectomy (ALPPS) performed in a patient with colorectal cancer liver metastasis. The current case report was written according to the SCARE criteria [2].
Case report
In July 2022, a 49-year-old female, otherwise healthy, presented symptoms of hematochezia and stool caliber changes. A colonoscopy was done, where an exophytic neoplastic lesion was detected on the mucosal surface, measuring around 8 × 3 cm, dark brown, friable and close to obstruction (approximately 70%) at the level of the sigmoid colon, a biopsy of that lesion, pathology reported a moderately differentiated invasive adenocarcinoma originated in the background of a colonic adenoma with high-grade dysplasia. A computed tomography (CT) scan was ordered showing a 7 × 3 cm mass in the sigmoid colon as well as a couple of lesions in the liver in segments 5, 6, 7 and 8, consistent with metastasis. A radical resection of the primary cancer with a left hemicolectomy was with primary anastomosis. After recovering from surgery, the patient was considered for systemic treatment based on eight cycles of oxaliplatin and capecitabine. A new CT scan showed two liver lesions measuring 5 and 6 cm, occupying segments 5, 6, 7, and 8, in addition to at least two other smaller lesions in segments 6 and 7, with no abnormal enhancement under contrast. A liver magnetic resonance imaging (MRI) (June 2023) showed the same lesions corresponding to metastatic deposits, conditioning dilation of the intrahepatic bile duct mainly on its right posterior branch (Fig. 1).
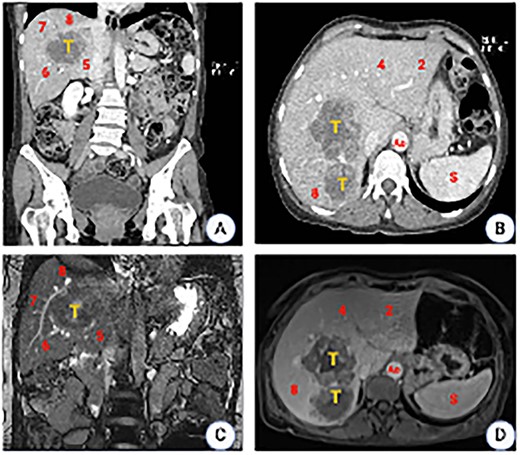
CT scan images. (A) Coronal plane showing the tumor location involving segments 5, 6, 7 and 8. (B) Axial plane showing the relation to the inferior vena cava (IVC). (C) MRI showing a liver mass with peripheral and heterogeneous central enhancement. (D) Axial MRI (T1phase). Ao, aorta; S, spleen.
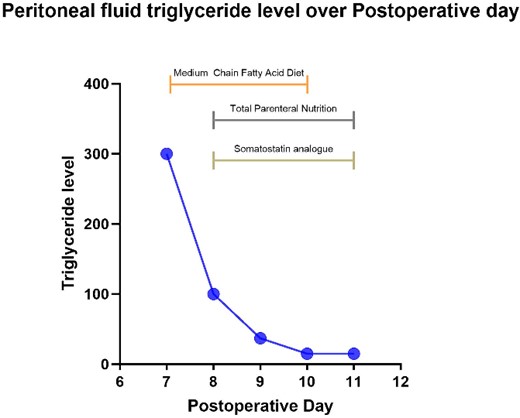
Prior to surgery, a 3D liver reconstruction image with liver volumetry was performed using a free open-source software (3D Slicer) (Fig. 2). Liver volumetry calculated the volume of the right lobe and segment 4 was 1401.2 cc (77% of the total volume) and the volume of segments 1, 2 and 3 was 418 cc (23% of the total volume), given the previous exposure to chemotherapy and an insufficient FLR with a future liver remanent/weight ratio (FLR/WR) of 0.44, it was decided to perform an extended right hepatectomy using the ALPPS technique (Fig. 3). It was calculated the Comprehensive ALPPS Preoperative Risk Assesment (CAPRA) score wich was of 3.96, with an estimated 90d mortality <7% and the pre-stage 1 ALPPS risk model of 0 points with an early mortality risk of 2.7%. The PO course was uneventful, until the PO Day 7, when the fluid drain turned milky, chylous ascites was confirmed by determination of triglyceride levels in the drainage fluid at 300 mg/dL; thus, management with medium chain fatty acids rich diet, parenteral nutrition and somatostatin analog (octreotide) was started. On PO Day 10 of treatment, there was evidence of decreased drainage volume and serous discoloration with a normalization of triglyceride fluid levels, so a normal diet was started with adequate tolerance, the liver function tests were normal at that time, and the patient was discharged on PO Day 11 (Fig. 4).
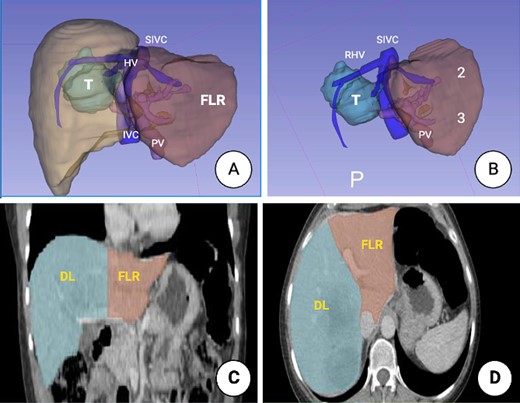
3D image reconstruction. (A) Right hemi-liver mass involving segments 5, 6, 7 and 8. The relation to the suprahepatic veins (HV), IVC and the PV is shown. (B) Future liver remnant (FLR) (Segments 2 and 3). SIVC, suprahepatic inferior vena cava; PV, portal vein; FLR, future liver remnant. (C) Liver volumetry. Coronal plane delimiting the transection line planned and the FLR. (D). Axial plane, showing the transection line planned and the FLR.
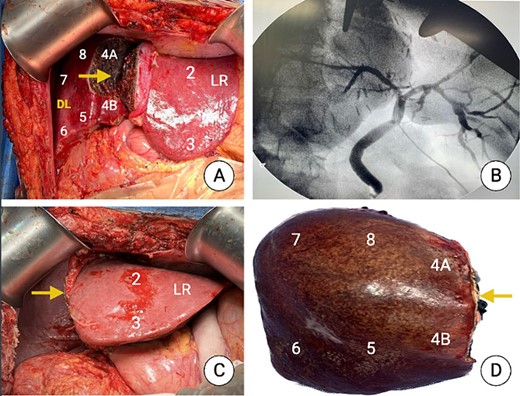
Intraoperative picture after the first stage (once completed the partial liver transection) and after the second stage. (A) Partial transection just to the right of the falciform ligament showing segments 2 and 3 (LR). (B) Intraoperative cholangiography. (C) Liver remnant (Segments 1, 2 and 3 at the end of the second stage). (D) Specimen after surgical removal showing the liver segments, and the transection plane (arrow).
Discussion
It is widely accepted that every patient presenting with colorectal liver metastasis should be evaluated by a hepatobiliary surgeon and discussed all the available institutional treatment options considering that all the resectable lesions should be resected. In our center, we have been using several liver growth techniques including two-stage hepatectomy, portal vein (PV) embolization, PV deprivation as well as ALPPS procedure. The lesion may occur in the cisterna chyli or in major lymphatic vessels; in most cases, they seal spontaneously, without clinical implications. During liver surgery, inadvertent injury to the lymphatic ducts in the hepatoduodenal ligament can cause chylous ascites [3]. Cytology and chemical analysis of the drainage fluid confirms the diagnosis by measuring serum triglyceride levels above 110 mg/dL; the presence of lymphocytes >80% also supports this diagnosis [4]. If the drainage is less than 100 ml/day, medium-chain triglycerides (MCT) therapy or a low-fat diet is recommended to avoid prolonged fasting. A few cases of management following chylous ascites have been reported. No series or case reports have been reported following ALPPS [5]. Kuboki’s group reported in a series of patients with chylous ascites some risk factors for its development, such as manipulation of the hepatoduodenal ligament, removal of the caudate lobe, resection of retroperitoneal tissue, manipulation of the para-aortic area, retroperitoneal invasion and early enteral feeding [6]. In a study by Assumpção et al., they found that the number of dissected lymph nodes and vascular resection were independent risk factors for chylous ascites in pancreatic resection and treatment with octreotide decreased drainage output on day one of its initiation [7].
The management of chylous ascites described after hepatectomy is based on fasting and total parenteral nutrition. Management without discontinuation of the oral intake has not been described, only in one reported case; it was based on a fat-free diet, supplemented with MCT, protein and octreotide, and removal of the abdominal drainage [8]. The initial treatment is controversial, which aimed to decrease the amount of chyle formation and reducing lymph flow. It is described in the reports as conservative based on strict fasting, parenteral nutrition and octreotide. To our knowledge, we report the first case of chylous ascites following extended right hepatectomy after the ALPPS procedure.
In conclusion, there is currently no international consensus for the standardization of therapy, so medical management based on a high-protein diet and fat restriction, supplemented with MCT, with or without parenteral nutrition and somatostatin analog, constitutes a reasonable treatment in order to avoid surgical treatment.
Conflict of interest statement
None declared.
Funding
None declared.



