-
PDF
- Split View
-
Views
-
Cite
Cite
Yohei Kameda, Hiroyuki Osawa, Yui Sueishi, Yoshihiro Ishikawa, Takamitsu Maehara, A case of delayed bleeding of the chest wall after VATS treated with transcatheter arterial embolization, Journal of Surgical Case Reports, Volume 2024, Issue 5, May 2024, rjae271, https://doi.org/10.1093/jscr/rjae271
Close - Share Icon Share
Abstract
We report a case of delayed bleeding after video-assisted thoracic surgery (VATS) that was successfully treated with transcatheter arterial embolization. An 81-year-old woman underwent a pleural biopsy via VATS for pleural dissemination of lung cancer. The postoperative course was good, but 8 days later she was hospitalized for swelling in the right axilla and was admitted to our hospital with a diagnosis of delayed postoperative hemorrhage. Gauze compression was performed, and the patient was discharged without exacerbation of hematoma. However, 4 days later, she was hospitalized for rapidly worsening swelling and pain. Chest computed tomography at the time of rebleeding showed an increase in the hematoma and extravasation in the peripheral right lateral thoracic artery. The patient was immediately treated with emergency angiography, and coil embolization was performed. After this treatment, the patient has done well and there has been no subsequent recurrence of bleeding.
Introduction
Delayed chest wall bleeding is a rare complication of video-assisted thoracic surgery (VATS). We report a case of delayed bleeding after VATS that was successfully treated with transcatheter arterial embolization (TAE).
Case report
An 81-year-old woman with suspected lung cancer of the right middle lobe with pleural dissemination underwent a pleural biopsy via VATS (Fig. 1). She also had chronic carotid artery stenosis and took clopidogrel, which was stopped 7 days before surgery. Before wound closure, hemostasis of the incision and port insertion site was confirmed by thoracoscopy. On the second day after surgery, she was discharged without any complications and oral administration of clopidogrel was resumed. Eight days later, she was hospitalized for swelling in the right axilla and was admitted to our hospital with a diagnosis of delayed postoperative hemorrhage (Fig. 1). Since her vital signs were stable and pain was mild, gauze compression was performed at night. Oral clopidogrel was discontinued. The next morning, the hematoma had not grown, so gauze pressure was continued for 2 days. She was discharged with no exacerbation.
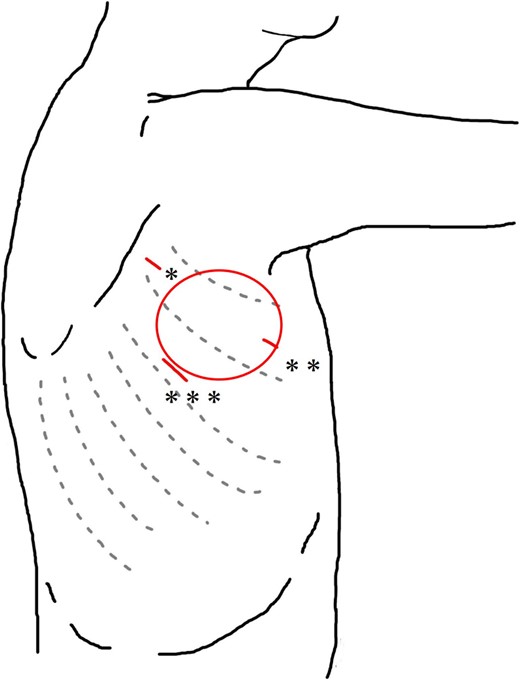
Schema of the surgical wound in VATS. *Third intercostal space on the anterior edge of the scapula. **Third intercostal space on the midaxillary line. ***Fifth intercostal space on the posterior axillary line. Circle: Location of swelling.
Four days later, the patient was hospitalized for rapidly worsening swelling and pain, and was readmitted with a diagnosis of rebleeding from the chest wall. Physical examination revealed a fist-sized subcutaneous mass on the right side of the chest, with severe pain. Laboratory data indicated anemia (Hb 7.8 g/dl) and no coagulation disorder: % prothrombin time (PT) 115.1%, prothrombin time-international normalized ratio (PT-INR) 0.93, and activated partial thromboplastin time 23.6 s. Computed tomography (CT) at the time of the first hemorrhage showed the hematoma on the right chest and anterior to the scapula (Fig. 2). Contrast-enhanced CT at the time of rebleeding showed an increase in hematoma and extravasation in the peripheral right lateral thoracic artery (Fig. 3). The patient was immediately treated with emergency angiography, which revealed active bleeding from a pseudoaneurysm of the right lateral thoracic artery. Thus, coil embolization was performed (Fig. 4). There were no TAE-related complications and the patient was discharged to home on the fifth hospital day. Oral clopidogrel was resumed and there has been no recurrence of bleeding.
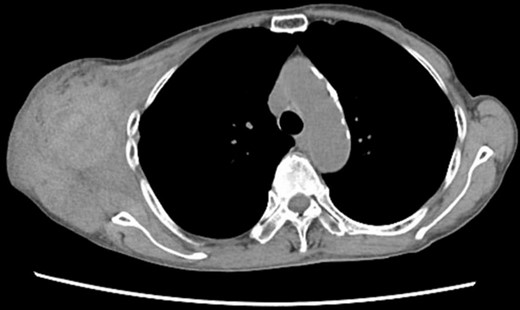
Chest CT at the time of the first bleeding showed formation of a hematoma on the right chest and front of the scapula.
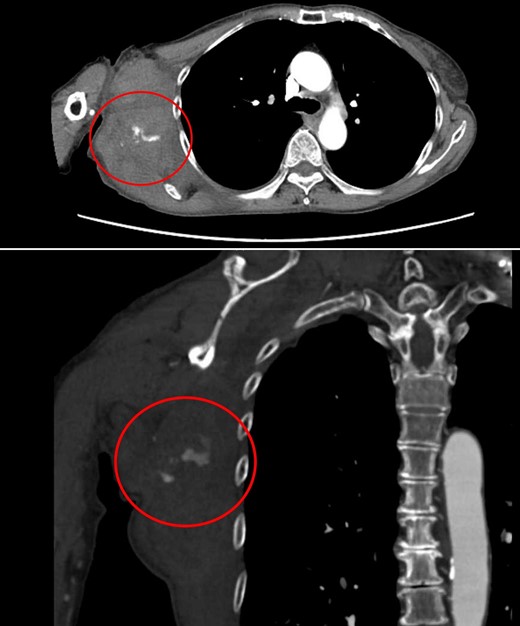
Contrast-enhanced CT at the time of the second bleeding showed growth of the hematoma and extravasation of contrast agent in a branch of the right lateral thoracic artery.
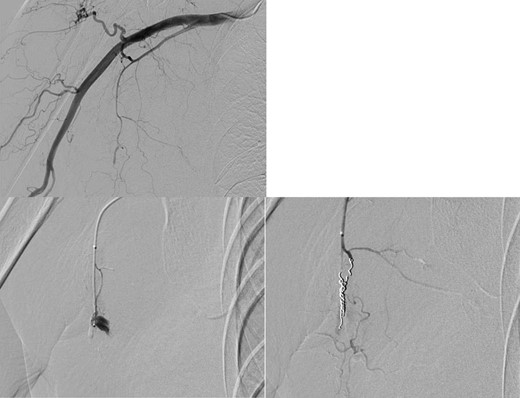
Angiography showed active bleeding from a pseudoaneurysm of the right lateral thoracic artery. Coil embolization was performed.
Discussion
Chest wall bleeding may occur due to blunt trauma [1] and after thoracentesis or insertion of a chest drainage tube [2–5]. Some cases only develop hematomas on the chest wall, while others develop hemothorax [6]. However, delayed bleeding of the chest wall after thoracic surgery is rare. There has been a report of a complication after valvular heart surgery with small thoracotomy [6], but complications after lung cancer surgery or thoracoscopic surgery have not been described. The lateral thoracic artery is a branch from the axillary artery that descends the chest wall and enters the serratus anterior muscle. Conventional thoracotomy such as posterolateral incision or axillary thoracotomy often involves dissecting this vessel, but the wide operative field allows sufficient hemostasis through ligation or cauterization. This may be one of the reasons why there are few case reports of delayed bleeding after thoracic surgery.
Use of VATS in general thoracic surgery is increasing, with the 2017 annual report of the Japanese Society of Thoracic Surgery finding use of this method in 73% of primary lung cancer surgeries and 94% of benign lung tumor surgeries [7]. VATS has been suggested to be superior or similar to conventional open surgery in terms of perioperative complication rates [8–11]. However, there are no large-scale studies comparing the incidence of delayed hemorrhage of the chest wall after VATS and thoracotomy. VATS has a smaller surgical field for treatment of the chest wall vasculature and the risk of bleeding may be higher.
In this case, when the operation was performed, the branch vessels of the lateral thoracic artery were coagulated and dissected immediately below the surgical wound, and hemostasis was obtained during the operation. However, 10 days after surgery, the patient developed delayed bleeding. This was thought to have been caused by the increase in body movement upon return to daily life after discharge and the resumption of oral antiplatelet drugs. Regarding the timing of delayed chest wall bleeding, Kamada et al. [6] found that hemothorax occurred 7 days after thoracic surgery, and Misthos et al. [12] reported a time from injury to onset of 2–14 days (mean 7 days) in 52 patients with delayed post-traumatic hemothorax. In this case, bleeding occurred 10 and 17 days after the operation. These findings suggest that careful follow-up for about 2 weeks is important for late complications after thoracoscopic surgery, especially in high-risk cases such as those of patients on anticoagulants and antiplatelet drugs.
As a method for hemostasis, we chose gauze compression for the initial postoperative bleeding, but this was insufficient and rebleeding occurred 17 days after the operation. Other treatments include hemostasis by endovascular intervention [6, 13–15] and surgical hemostasis. In this case, we chose endovascular treatment, since this is less invasive than reoperation, the bleeding site was identifiable on angiography, and risks of general anesthesia, wound infection and lung injury in reopening surgery were avoided. The outcome suggests that angiography may be a better treatment method, especially in cases with unstable vital signs.
We experienced a case of delayed hemorrhage of the chest wall after VATS. This case indicates that patients taking anticoagulants or antiplatelets should be carefully monitored after surgery, and that TAE is an effective method for hemostasis.
Conflict of interest statement
None declared.
Funding
None declared.



