-
PDF
- Split View
-
Views
-
Cite
Cite
Ruoyue Hua, Jun Zhong, Jianlin Xian, Yaoqi Liang, Zilin Gan, Shoupeng Deng, Perforation of descending colonic cancer as a rare cause of gas gangrene of the lower limb in an 80-year-old female: a case report, Journal of Surgical Case Reports, Volume 2024, Issue 4, April 2024, rjae033, https://doi.org/10.1093/jscr/rjae033
Close - Share Icon Share
Abstract
Gas gangrene is a rare, severe gas-producing infection that can be related to colorectal cancer. Gas gangrene can be confirmed by radiologic findings and crepitation on touch. Spontaneous gas gangrene can be associated with colorectal cancer. An 80-year-old female complaint about a sudden abdominal pain, accompanied with progressive swelling pain in thigh and fever. Diagnosis based on assessment findings were gas gangrene and descending colonic cancer perforation. Emergency surgery was performed for debridement and drainage, followed by vacuum sealing drainage (VSD) with polyurethane (PU). Two more surgical interventions were given before the colonic tumor surgery. The patient recovered well in the long-term follow-up. This report demonstrates the diagnosis, treatment, and management of a successful case of gas gangrene caused by perforation of descending colonic cancer. Accurate preoperative diagnosis and reasonable use of VSD (PU) material played an important role in the treatment of this case.
Introduction
Gas gangrene, also known as (clostridial) myonecrosis, is a rare, highly lethal gas-producing necrotizing infection of muscle [1, 2] characterized by severe, rapidly developing infections of deep soft tissue, threats to limb and life [3, 4] and poor prognoses [5]. Spontaneous or atraumatic gas gangrene arises without any external injury and is usually related to cancer and immunosuppression [1]. Among the clostridial organisms causing infections, Clostridia septicum is responsible for spontaneous gas gangrene more often [2, 6–8]. It is mentioned in previous studies that spontaneous gas gangrene caused by Clostridium septicum infection can be associated with bowel perforation and colorectal cancer [2, 3, 5, 6, 9]. Although rare, non-clostridial gas gangrene can also be present [10]. For patients with colonic cancer, perforation of colon is a rare complication. It tends to occur in older patients [11] and can form an ideal portal of organism, initiating infection.
In this case report, we present a rare case of spontaneous gas gangrene caused by perforation of descending colonic cancer in an 80-year-old female patient. The patient was admitted to and treated in a 3A-level general hospital in Wuzhou, China.
This case has been reported in line with The SCARE 2020 Guideline: Updating Consensus Surgical CAse REport (SCARE) Guidelines [12].
Presentation of case
An 80-year-old female patient was brought to the emergency room on a wheelchair. The patient complained of a sudden, persistent pain in the left lower abdomen, which lasted for three days and gradually developed severe enough to limit movement and spreaded to the left groin and left thigh, accompanied by chills and high fever (~39.5°C). Since the onset, the patient had been experiencing poor appetite, sleep, and mental state, with less urination and no excrement. At local clinic she received Traditional Chinese Medicine treatment which was barely effective. In the last six months, she had often felt weak, thinner and been experiencing left lower abdominal discomfort and pain with abnormal excrement.
Initial assessment findings included a temperature of 39°C, a heart rate (HR) of 108 bpm, and a blood pressure (BP) of 86/60 mm Hg. Left lower abdomen were slightly distended, where deep palpation can reach a mass of about 15*10 CM with an ill-defined boundary and fixed pain, which was confined to the left lower abdomen. A negative shifting dullness was found. Bowel sounds were 4–6 times/min. Skin and subcutaneous tissue in the left groin were visibly more distended than the opposite side, without obvious skin rupture and subcutaneous ecchymosis. This area revealed crepitation on touch. The left thigh was also obviously swollen and distended, with slightly higher skin temperature, distinct pain, and palpable crepitation on touch. The patient was fully conscious and aware of surroundings.
Laboratory examination demonstrated a white blood cell (WBC) count of 32.42*109/L (3.50–9.50*109/L), a procalcitonin (PCT) level of 4.23 ng/ml (<0.05 ng/ml), a hypersensitive C-reactive protein level of >160 mg/L (0.0–5.0 mg/L), and a carcinoembryonic antigen (CEA) level of 136.38 ng/ml (0.0–5.0 ng/ml). A computer tomography (CT) scan revealed swollen, blurred soft tissue with pneumatosis in left psoas major, left lower back, left pelvic cavity, left pelvic wall, left groin region, left thigh—upper left calf, and bowel (probably descending colon) wall thickening and calcification in left lower abdomen accompanied with slightly narrowed intestinal lumen in the same segment (Fig. 1). According to the clinical features, principal diagnosis were gas gangrene in the left groin and left thigh and descending colonic cancer perforation with left lower abdominal abscess.
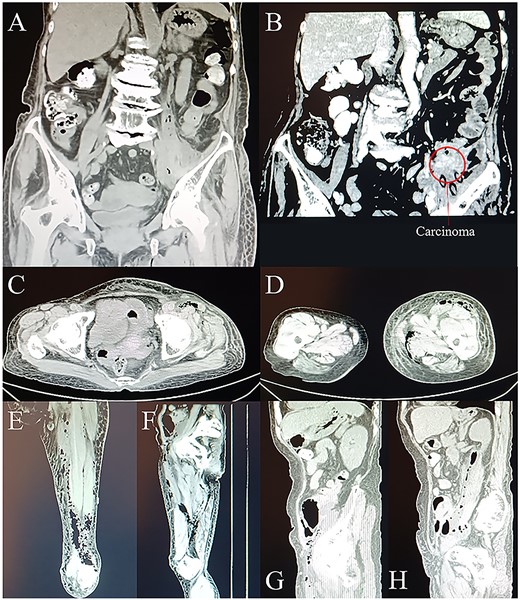
CT scans on day 1. (A) and (B) Coronal planes from CT display abdominal gas formation, abscess and tumor around descending colon. Tumor locates in the left lower abdomen. (C) Transverse, (E) coronal, and (G) sagittal planes from CT display gas extending to and going through the groin area. (D) Transverse and (F) coronal planes from CT display the downward route of gas formation. (H) Sagittal plane from CT displays the involvement of iliopsoas muscle.
Due to the patient’s severe symptoms of infection and low blood pressure on admission, despite the temporarily unavailable bacterial culture result, emergency surgical expansion and drainage were performed, giving priority to saving lives. Considering Escherichia coli and C. septicum as the main gas-producing bacteria in the context of descending colonic cancer perforation, meropenem (0.5 g q8h) was given as a third-line therapeutic for anti-infection treatment. Other adjuvant therapies were given to maintain blood pressure, protect organ function, correct low protein, maintain electrolyte balance, provide nutritional support, and prevent various related complications.
The first surgery was performed several hours after admission, aiming to debride necrotic tissue and pus for infection control, and to take tumor specimens for pathological examination. Multiple longitudinal incisions were made for drainage to avoid the perforators being damaged, which may lead to extensive skin necrosis in the blood supply area. During the surgery, a large amount of pus and gas overflowed from under the skin of the whole thigh and each intermuscular space with distinct stench of dung (Fig. 2A). Extensive necrosis was found in the subcutaneous fat fascia and the intermuscular fascia. Pus samples were taken for drug sensitivity test and bacterial culture. Blunt dissection was performed carefully and necrotic tissue under direct vision was excised, followed by repeated irrigation with huge amount of hydrogen peroxide and normal saline.
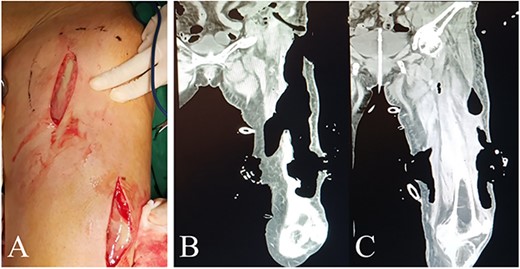
Intraoperative view and postoperative CT of left thigh in the first operation. (A) Intraoperative anterior view of left thigh. (B) and (C) Coronal planes from CT 7 days after the first operation display areas dressed with VSD (PU) material.
An infection pathway of about 3*2 CM at the inguinal posterior peritoneum invagination into the posterior peritoneal cavity was found and then widen by about 2 CM. The posterior peritoneal cavity was also irrigated with hydrogen peroxide and normal saline repeatedly using syringes in order not to injure peripheral vessels. Large quantity of pus was drained without distinct fecal residue, so we considered a small pore size of intestinal perforation. Pathological examination of posterior peritoneum was abandoned for limited vision. The drainage tubes were placed, and polyurethane (PU) were positioned as vacuum sealing drainage (VSD) material in the intermuscular spaces of left thigh and groin, and the posterior superficial peritoneum, respectively (Fig. 3). The wounds were sealed with films and connected to a vacuum source for creating negative pressure.
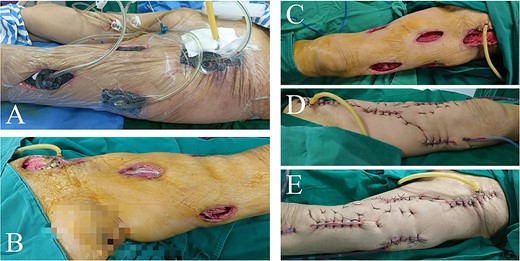
Intraoperative view of left thigh in the second and third operation. Lateral view of left thigh before (A) and after (B) removing VSD (PU) material in the second operation. (C) Anterior view of left thigh after removing VSD (PU) material in the third operation. (D) Interior and (E) lateral view of repaired left thigh.
Postoperative findings of laboratory examination improved significantly. The patient spent several days in residential care in surgical ward with nutritional support. Bacterial culture of pus extracted in the first surgery was negative. On hospital day 12, a second surgery was performed to remove VSD (PU) material, finding a large amount of remaining necrotic fascia and part of the pus cavity not completely cleared. After thorough debridement, the left thigh and the posterior peritoneal pus space were treated with VSD and filled with PU again. Significant improvement were observed in symptoms and laboratory findings, so meropenem was discontinued and downgraded to amoxicillin on day 16. In the third surgery on day 19, distinct fresh wounds without necrotic tissue were observed. The drainage tubes were placed in multiple places to fully drain the potential remnant cavity in the thigh. Multipoint skins were sewn and fixed, and then received pressure dressing with self-adhesive elastic bandage. The abdominal drainage tube was retained and kept unobstructed for subsequent tumor surgery.
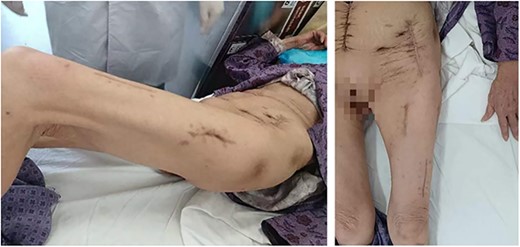
The patient was transferred to the gastrointestinal department for another colonic cancer surgery on day 30. Postoperative pathology supported our diagnosis. The patient received following treatment in local hospital and returned for follow-up 11 months later. She came on her own foot in a good mental state with an increased body weight of about 5 kg than 6 months ago (Fig. 4).
Discussion
Diagnosis and treatment of spontaneous gas gangrene remain clinical challenges due to the endogenous nature, high mortality, extreme age of patients, nonspecific symptoms, and rapid fulminating course, etc [1]. No consensus have been reached yet about the diagnostic criteria and diagnosis relies largely on radiological findings of gas retention in affected area [5]. Diagnoses of gas gangrene should be considered when severe body infection, subcutaneous gas shown by imaging and crepitus during physical examination are present. In this case, the patient had no visible infection on admission to hospital, therefore differential diagnoses of necrotizing fasciitis, cellulitis and other acute abdominalgia were essential for following management. Necrotizing fasciitis can destroy subcutaneous tissue and deep fascia layers, hence surgical management should continue daily until all necrotic tissue have been removed [13]. Cellulitis is relatively benign and can be controlled with antimicrobial treatment [14]. Non-surgical treatment is preferred for acute abdominalgia. As for treating gas-producing limb infections, immediate surgery is the fundamental measure [15]. Complete debridement within 12 h after onset can significantly reduce the mortality of patients [16]. We made accurate, prompt diagnosis on history-taking, physical examination and relevant ancillary tests with years of clinical experience in general surgery. The etiology of descending colonic cancer perforation was also proposed early. Timely surgeries were given and the patient’s limb and life were saved. The accurate preoperative diagnosis played an important role in guiding the surgical intervention and postoperative treatment.
We suppose that reasonable use of VSD (PU) material was the key to treatment of this case. Surgical debridement combined with VSD can drain local necrotic tissue and exudate more efficiently, increase the blood supply of the wound and promote the growth of granulation tissue. Due to the large surgical wounds and the different degrees of necrosis in intermuscular fascia of the left thigh, daily dressing change may cause painful shocks in the patient and uncomplete dressing change resulting in remnant cavities. To avoid these potential negative effects on later surgeries, all the VSD (PU) materials were replaced in the operating room under anesthesia. After removing the previous dressing, further debridement were given and newly appropriately cut VSD (PU) materials were inserted, with care taken to avoid directly covering the deep large blood vessels and nerves and the unpredictable bleedings.
Regrettably, the bacterial culture was conducted only once in the whole process and no positive results were found. The authors have experienced an amount of clinical cases where almost all clinical features (including medical history, symptoms, and imaging findings) met the diagnosis criteria for gas gangrene, yet the laboratory culture result for anaerobic bacteria was hardly positive. Brucato et al. [17] studied 25 cases that were initially diagnosed as gas gangrene with palpable crepitus and subcutaneous or intermuscular gas accumulation revealed by radiographic examination. No clostridium was detected in microbial culture. This indicates that some gas-producing infections usually diagnosed as “gas gangrene” can also be caused by non-Clostridium species, and non-clostridium gas gangrene should be diagnosed in these cases. Proteus, Enterococcus, and E. coli can be the sources of gas-producing infections. Despite the lack of support with laboratory findings and unconfirmed diagnosis of gas gangrene, it is recommended by previous studies that treatment should not await and immediate surgical exploration and debridement should be undertaken [1, 4, 9]. An urgent and appropriate surgical intervention was given as recommended and infections were successfully controlled. Our diagnoses were confirmed in the following postoperative pathology of gastrointestinal surgery.
Conclusions
Spontaneous gas gangrene is a rare, severe infection threatening limb and life. This report demonstrated a successful case of gas gangrene related to descending colonic cancer. This rare case indicates that gas gangrene can present as non-clostridial and hence negative for Gram-positive bacilli. Gold standard for accurate preoperative diagnosis and reasonable use of VSD (PU) material played an important role in the treatment of this case.
Author contributions
Conception or design: R.H.
Acquisition, analysis, or interpretation of data: J.Z., J.X., Y.L., Z.G., S.D.
Drafting the work or revising: R.H.
Final approval of the manuscript: R.H.
Conflict of interest statement
The authors have no conflicting interests to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data used to support the results of this study can be obtained from the corresponding author upon request.
Ethical approval and consent to participate
This study was approved by the Medical Ethics Committee of our hospital. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.



