-
PDF
- Split View
-
Views
-
Cite
Cite
Lauren Wallace, Peter J Gallagher, A diagnostic dilemma: a case report of concomitant duodenal Dieulafoy lesion and gastric ulcer, Journal of Surgical Case Reports, Volume 2024, Issue 3, March 2024, rjae122, https://doi.org/10.1093/jscr/rjae122
Close - Share Icon Share
Abstract
Dieulafoy lesions (DL) are an uncommon cause of gastrointestinal bleeding which is often difficult to diagnose due to the rarity of the condition and varying clinical presentations. This case describes an unusual presentation of upper gastrointestinal bleeding in an 85-year-old female with findings on two separate gastroscopies of both a gastric ulcer and duodenal DL. The pathophysiology of DL remains poorly understood and despite shared risk factors, these two pathologies are rarely reported concurrently. The presence of a concomitant gastric ulcer further complicated the diagnosis and treatment of the duodenal DL in this case. This highlights the importance of clinician awareness of this pathology and its presentation and the need for early repeat endoscopy.
Introduction
A Dieulafoy lesion (DL) is an exposed aberrant submucosal artery without surrounding ulceration, most commonly found in the stomach [1]. It is an uncommon source of gastrointestinal bleeding (GIB), with a propensity to cause large volume haemorrhage, severe anaemia and shock [2]. Despite advances in endoscopic techniques, the diagnosis of DLs remains difficult [1, 3]. This is likely due to the varying presentations and intermittent bleeding of DLs, in combination with the infrequency of the condition and a lack of clinician familiarity [1, 4]. This case explores the challenges of diagnosing a duodenal DL in the context of a concurrent gastric ulcer.
Case report
An 85-year-old female presented to hospital with melena and anaemia. She was functionally independent. Her past medical history included hypertension, and medications included irbesartan and low dose aspirin for primary cardiovascular prevention. She was haemodynamically stable with a haemoglobin of 75 g/L (previously normal) and urea of 21.9 mmol/L. CT-angiogram (CT-A) demonstrated no active contrast extravasation. She received two units of packed red blood cells (PRBCs), was commenced on intravenous pantoprazole, and underwent gastroscopy. This revealed a linear ulcer on the greater curvature of the stomach 5 cm from the gastroesophageal junction (GOJ) with an adherent clot and no active bleeding (Figs 1 and 2). The ulcer was injected with adrenaline and haemostasis achieved. The first and second part of the duodenum (D1, D2 respectively) appeared normal.
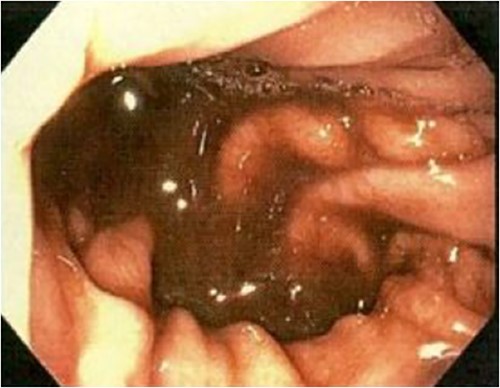
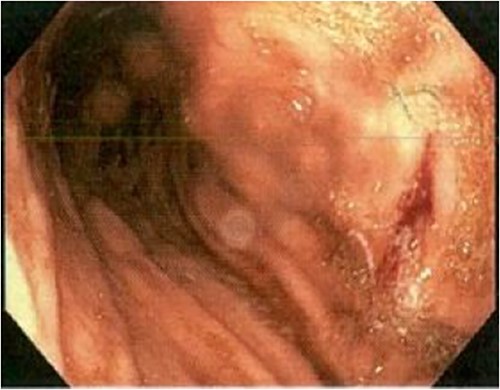
Initial gastroscopy - linear gastric ulcer on greater curvature of stomach.
Three days after initial gastroscopy she developed further melena and anaemia, requiring additional PRBCs. She underwent a second gastroscopy, which demonstrated a lesion consistent with a DL at the junction of D1/D2 which was actively bleeding from an exposed vessel (Fig. 3). Haemostasis was achieved with thermocoagulation and adrenaline injection (Fig. 4). The melena resolved and haemoglobin remained stable. The remainder of her admission and 2-month follow-up were unremarkable.
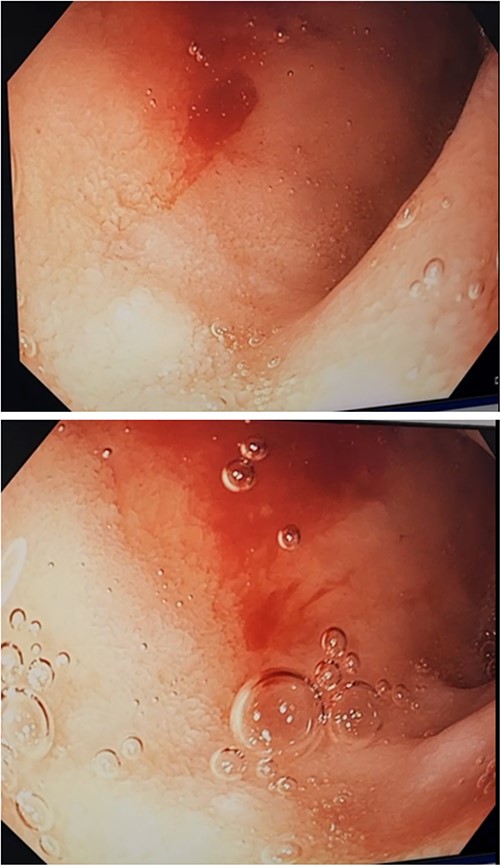
Second gastroscopy – active arterial bleeding at D1/D2 from small mucosal defect without surrounding ulceration.
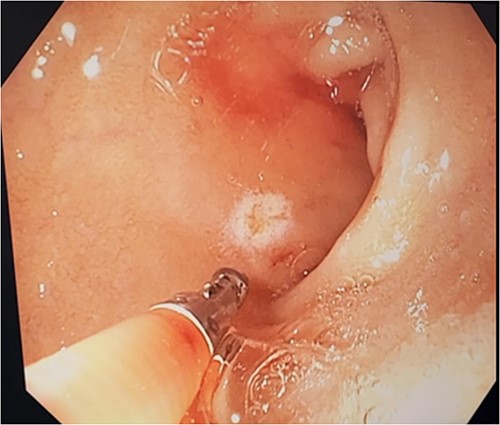
Second gastroscopy – successful haemostasis of actively bleeding duodenal DL with thermocoagulation and adrenaline injection.
Discussion
A DL is an anomalous submucosal artery which fails to narrow distally, maintaining a 10-fold larger diameter than other normal submucosal vessels [1, 5]. The artery is histologically normal and protrudes through a small mucosal defect with normal surrounding mucosa without ulceration [1, 3]. DLs are uncommon, estimated to account for 0.1%–2% of all upper gastrointestinal bleeding (UGIB) [1–3, 5, 6]. Up to 74% of DLs occur in the stomach with 61%–65% found on the lesser curve within 6 cm of the GOJ [1, 3, 5]. Duodenal DLs are rare, representing only 15% of reported cases [3, 7, 8]. Other rare sites reported include the jejunum [8], ileum [9], oesophagus [10], colon [11], and rectum [12].
The pathophysiology of DLs remains poorly understood. An association between DLs and advanced age, most commonly occurring in the fifth to seventh decades, and various co-morbidities, including chronic kidney disease, hypertension, and cardiovascular disease, suggests it is at least partially acquired [1, 2, 5, 6, 13]. However, reports of DLs in paediatric populations complicates these conclusions, suggesting an inherited component [14]. Proposed theories for arterial erosion leading to GIB include repetitive mechanical trauma by food on weakened mucosa, pressure of arterial pulsation from the larger artery on the mucosa, hypoperfusion, and ischemia from blood diversion and potential arterial thrombosis and necrosis [3, 5]. Additionally, mucosal atrophy and weakened vessels due to vascular disease in elderly populations may explain the increased presentations in this age group [1, 5].
Similarly, risk factors associated with DLs are incompletely characterized. Non-steroidal anti-inflammatory drugs (NSAIDS) and antiplatelet agents have also been strongly associated with DLs, with up to 50% of DL related UGIB associated with these agents [1, 2, 6, 8, 13]. No association has been demonstrated between DLs and Helicobacter pylori [1]. Despite these shared risk factors there are limited reports of concurrent gastric or duodenal ulcers and DLs as seen in this case. While some authors have suggested NSAIDs may play a role in contributing to DL rupture via a similar mechanism to gastritis and ulceration [13], others have proposed it plays a less important role in DL haemorrhage given the relatively shallower and larger calibre of the DL vessel [2].
Endoscopy is considered the gold standard diagnostic technique, allowing for immediate treatment [1, 5, 7]. While endoscopy has significantly improved survival for DLs, diagnosis remains a challenge with one study reporting only 49% of DLs were identified on initial endoscopy [2–4, 7]. Factors which contribute to this include the intermittent nature of bleeding, small lesion size with an absence of abnormal surrounding mucosa, and a lack of clinical experience with the condition [1, 4, 7, 8]. Additionally, it may further be obscured by pooling of blood or gastric contents [4, 7]. Duodenal angulations, folds and diverticulum may also further conceal DLs in the duodenum [7, 8]. This case was also complicated by the presence of a concurrent gastric ulcer, which further emphasizes the need for recognition of DL presentations and consideration of other pathologies with shared risk factors. CT-A has been reported to aid in diagnosis of gastric DLs, seen as an abnormally large vessel or non-specific contrast blush in the submucosa with or without active arterial extravasation [5, 15]. However, initial CT-A in the current case failed to reveal such characteristics. It is possible, duodenal DLs are more difficult to identify on CT-A or appear differently.
Endoscopic treatment is the mainstay of DL management, with surgery and angioembolisation reserved for those with instability or failed endoscopic treatment [1, 8]. Various endoscopic options have been described, with success rates of up to 96% [2, 6–8]. However, there is a lack of consensus on optimal treatment modalities [1, 7]. DL-related UGIB has increased rates of rebleeding and readmission compared to other causes, which is important to consider when choosing a treatment [2, 6]. Techniques reported include adrenaline or cyanoacrylate, mechanical therapy with banding or haemoclips, and thermocoagulation [5, 7]. Studies suggest combination therapy reduces rebleeding, particularly when compared to adrenaline alone with reported rebleeding rates of up to 32.7% [1, 5, 8]. Some authors suggest improved efficacy with mechanical therapies [6–8], however combination therapy with adrenaline and thermocoagulation also appears to be an effective alternative [1, 5, 8]. In line with prior reports [4, 7, 8], this case demonstrates successful primary haemostasis and prevention of rebleeding of a duodenal DL with combination thermocoagulation and adrenaline injection.
Conclusion
While DL is an uncommon cause of UGIB, it should be considered in those presenting with intermittent GIB even in the presence of a concurrent aetiology. A high index of suspicion is required for accurate diagnosis and treatment, and the importance of repeat endoscopy cannot be understated.
Conflict of interest statement
The authors declare no conflicts of interest relevant to this article.
Funding
No funding source for this article.



