-
PDF
- Split View
-
Views
-
Cite
Cite
Qusay Abdoh, Enas Samara, Alaa Zayed, Mohammed Khader, Iyad Maqboul, Mohammed A M Rashed, Dieulafoy lesion with rare vascular complications: diagnostic and therapeutic approach, Journal of Surgical Case Reports, Volume 2024, Issue 12, December 2024, rjae750, https://doi.org/10.1093/jscr/rjae750
Close - Share Icon Share
Abstract
Dieulafoy lesions are a rare cause of gastrointestinal bleeding, characterized by an enlarged submucosal blood vessel that bleeds without visible abnormalities. The diagnosis is typically made via endoscopy, and treatment usually involves endoscopic therapy. This case involves a 46-year-old female who presented with upper gastrointestinal bleeding due to a Dieulafoy’s lesion, treated with band ligation and later embolization after the lesion was found to originate from the left phrenic artery. The patient developed rare complications, including splenic infarction and pleural effusion, which required additional management. Upon initial investigations, the patients had an accidental finding on computed tomography of asymptomatic celiac artery compression that was advised to take into consideration for the possibility of developing median arcuate ligament syndrome and was treated conservatively. The case highlights the diagnostic and therapeutic challenges of Dieulafoy’s lesion and underscores the need for individualized treatment and further research.
Introduction
Dieulafoy lesion is a rare cause of gastrointestinal bleeding, accounting for <2% of all cases [1]. It is an enlarged submucosal blood vessel that bleeds in the absence of any abnormality, such as ulcers or erosions, commonly found in the stomach but can occur in other parts of the gastrointestinal (GI) tract [2]. The pathophysiology of Dieulafoy’s lesion is unclear, but it is thought that bleeding comes from erosion of an abnormally protruding dilated vessel beneath the mucosa; mechanical stress or ischemic injury may be involved in vessel erosion [1]. The gold standard for diagnosing Dieulafoy lesions is endoscopy. Despite their mild nature, they can cause life-threatening bleeding. Endoscopic therapy is the first-line treatment [2].
Dieulafoy lesions are a rare cause of upper gastrointestinal bleeding; patients typically present with intermittent, acute, and severe gastrointestinal bleeding, which may manifest as hematemesis or melena [2]. These lesions are often under-recognized, and diagnosis is challenging, so understanding the differential diagnosis is crucial. Common causes of upper GI bleeding include peptic ulcer disease and esophageal varices, which are typically related to liver cirrhosis. Less frequent but important causes include Mallory–Weiss’s tears. Early endoscopy is essential for accurate diagnosis and effective treatment [3].
In this case report, we describe a 46-year-old female who presented with upper gastrointestinal bleeding secondary to a Dieulafoy’s lesion. Despite initial treatment, she developed complications, including splenic infarction and left pleural effusion. This case is significant for several reasons: the combination of these complications associated with a gastric vascular lesion is rare, presenting diagnostic and therapeutic challenges. Additionally, it enhances our understanding of managing such complex presentations and contributes to the broader clinical knowledge.
Case presentation
A 46-year-old female with no significant medical or surgical history presented with hematemesis and melena. Initial labs at a local center showed hemoglobin at 13.9 g/dl. Despite discharge on omeprazole 40 mg daily, melena persisted. She had no history of gastrointestinal disorders, bleeding tendencies, cardiovascular disease, smoking, alcohol use, or nonsteroidal-anti-inflammatory drugs (NSAID)/anticoagulant use.
She was referred to a secondary hospital due to the persistence of symptoms and stayed for 4 days, where she received an IV-esomeprazole infusion for 72 h, followed by oral proton-pump inhibitors (PPI) at 40 mg twice daily for 1 month.
During her stay, she received two units of packed red blood cells after her hemoglobin dropped to 6.5 g/dl. She was referred to our hospital, where an upper GI endoscopy revealed a bleeding Dieulafoy’s lesion (Fig. 1a), treated with band ligation (Fig. 1b and c). Continued medication and an abdominal computed tomography (CT) with IV contrast were recommended due to the risk of recurrent bleeding.
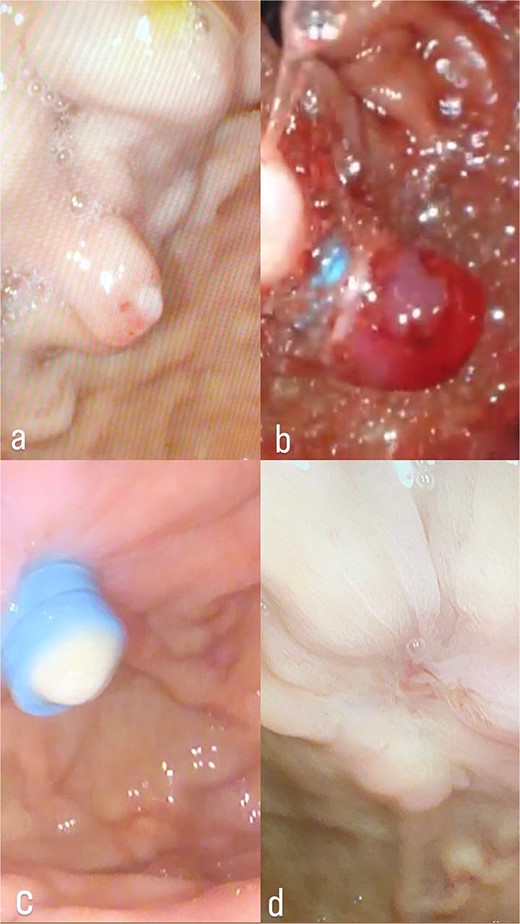
(a) Dieulafoy’s lesion, before ligation. (b) Dieulafoy’s lesion, immediately after ligation. (c) Dieulafoy’s lesion, after ligation. (d) Follow-up upper endoscopy was clear, with no bleeding or ulcers.
Upon admission to our hospital, the patient was alert and oriented. Her vital signs and initial laboratory tests are shown in Tables 1 and 2, respectively.
| Heart rate: 147 |
| Blood pressure: 113/68 |
| Temperature: 36.4°C |
| Respiratory rate: 18 |
| O2 saturation: 96%, on room air |
| Heart rate: 147 |
| Blood pressure: 113/68 |
| Temperature: 36.4°C |
| Respiratory rate: 18 |
| O2 saturation: 96%, on room air |
| Heart rate: 147 |
| Blood pressure: 113/68 |
| Temperature: 36.4°C |
| Respiratory rate: 18 |
| O2 saturation: 96%, on room air |
| Heart rate: 147 |
| Blood pressure: 113/68 |
| Temperature: 36.4°C |
| Respiratory rate: 18 |
| O2 saturation: 96%, on room air |
| Parameter . | Value . | Reference ranges . |
|---|---|---|
| Hemoglobin level | 10.2 g/dl | 12–16 g/dl |
| White blood cell count | 6.94 × 109/L | 4.5–11.00 × 109/L |
| C-reactive protein (CRP) | 41 mg/dl | 0.8–1.0 mg/dl |
| PLT | 254 | 150–400 |
| INR | 1.15 | <1.1 |
| PT | 15 s | 11–13 s |
| PPT | 30 s | 60–70 s |
| CR | 0.5 mg/dl | 0.5–1.10 mg/dl |
| BUN | 2 mg/dl | 8–20 mg/dl |
| Mg | 1.65 mg/dl | 1.7–2.2 mg/dl |
| Na | 137 mEq/L | 136–145 mEq/L |
| K | 3.6 mEq/L | 3.5–5.0 mEq/L |
| Cl | 105 mEq/L | 98–106 mEq/L |
| Parameter . | Value . | Reference ranges . |
|---|---|---|
| Hemoglobin level | 10.2 g/dl | 12–16 g/dl |
| White blood cell count | 6.94 × 109/L | 4.5–11.00 × 109/L |
| C-reactive protein (CRP) | 41 mg/dl | 0.8–1.0 mg/dl |
| PLT | 254 | 150–400 |
| INR | 1.15 | <1.1 |
| PT | 15 s | 11–13 s |
| PPT | 30 s | 60–70 s |
| CR | 0.5 mg/dl | 0.5–1.10 mg/dl |
| BUN | 2 mg/dl | 8–20 mg/dl |
| Mg | 1.65 mg/dl | 1.7–2.2 mg/dl |
| Na | 137 mEq/L | 136–145 mEq/L |
| K | 3.6 mEq/L | 3.5–5.0 mEq/L |
| Cl | 105 mEq/L | 98–106 mEq/L |
| Parameter . | Value . | Reference ranges . |
|---|---|---|
| Hemoglobin level | 10.2 g/dl | 12–16 g/dl |
| White blood cell count | 6.94 × 109/L | 4.5–11.00 × 109/L |
| C-reactive protein (CRP) | 41 mg/dl | 0.8–1.0 mg/dl |
| PLT | 254 | 150–400 |
| INR | 1.15 | <1.1 |
| PT | 15 s | 11–13 s |
| PPT | 30 s | 60–70 s |
| CR | 0.5 mg/dl | 0.5–1.10 mg/dl |
| BUN | 2 mg/dl | 8–20 mg/dl |
| Mg | 1.65 mg/dl | 1.7–2.2 mg/dl |
| Na | 137 mEq/L | 136–145 mEq/L |
| K | 3.6 mEq/L | 3.5–5.0 mEq/L |
| Cl | 105 mEq/L | 98–106 mEq/L |
| Parameter . | Value . | Reference ranges . |
|---|---|---|
| Hemoglobin level | 10.2 g/dl | 12–16 g/dl |
| White blood cell count | 6.94 × 109/L | 4.5–11.00 × 109/L |
| C-reactive protein (CRP) | 41 mg/dl | 0.8–1.0 mg/dl |
| PLT | 254 | 150–400 |
| INR | 1.15 | <1.1 |
| PT | 15 s | 11–13 s |
| PPT | 30 s | 60–70 s |
| CR | 0.5 mg/dl | 0.5–1.10 mg/dl |
| BUN | 2 mg/dl | 8–20 mg/dl |
| Mg | 1.65 mg/dl | 1.7–2.2 mg/dl |
| Na | 137 mEq/L | 136–145 mEq/L |
| K | 3.6 mEq/L | 3.5–5.0 mEq/L |
| Cl | 105 mEq/L | 98–106 mEq/L |
The CT scan confirmed a Dieulafoy’s lesion from the left phrenic artery (Fig. 2a and b) and showed collateral vascular channels around the pancreas with near-complete celiac trunk compression by the median arcuate ligament (Fig. 3). She underwent left phrenic artery embolization without complications, while asymptomatic celiac artery compression was treated conservatively. A follow-up endoscopy showed no bleeding or ulcers (Fig. 1d). Later, she developed severe left flank pain, and a contrast-CT revealed splenic vein thrombosis with infarction (Fig. 4), which was treated conservatively. Additionally, an incidental finding of left pleural effusion was drained. Rising inflammatory markers were managed with analgesics and IV antibiotics. She was discharged with outpatient follow-up, and at her 10-day visit, she reported improvement, stable hemoglobin, and normal inflammatory markers. She was satisfied, and a follow-up endoscopy was planned in 2 weeks.
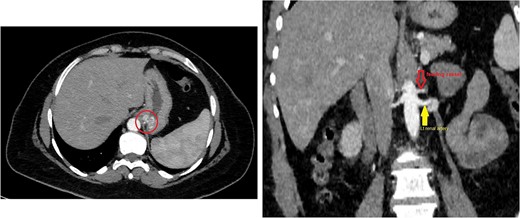
(a) Axial CT angio of the abdomen shows a small area of enlarged tortuous blood vessels seen along the posterior cardia of the stomach; these findings are suggestive of Dieulafoy lesion. (b) Coronal abdominal CT images show the feeding blood vessel arising from the descending abdominal aorta located just superior to the origin of the left renal artery.
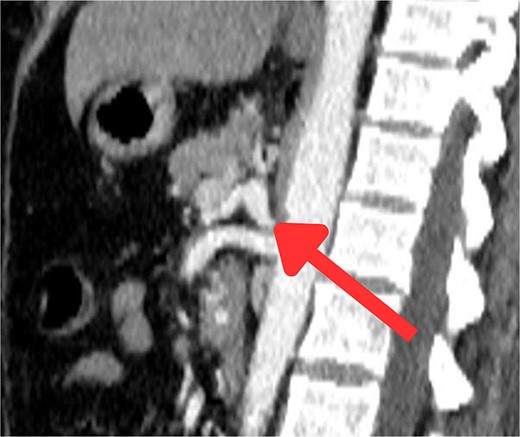
Sagittal abdominal CT angio shows complete obliteration of the celiac trunk due to compression from the medical arcuate ligament.
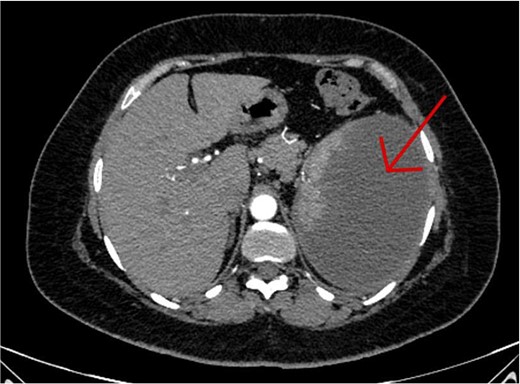
An axial CT scan of the abdomen shows enlarged spleen with almost total liquefaction of splenic parenchyma. Findings are in keeping with splenic infarction.
Discussion
A Dieulafoy lesion is an enlarged submucosal vessel, often near the gastroesophageal junction, responsible for 6.5% of upper gastrointestinal bleeds. It is more common in elderly men with cardiovascular disease or diabetes. Usually asymptomatic, it can cause melena, hematemesis, or anemia. Bleeding is related to artery pulsations, mucosal wear, or age-related atrophy [2]. In our case, the absence of risk factors like NSAID use, cardiovascular issues, or smoking, along with persistent symptoms despite PPI treatment, points to an atypical bleeding source.
Endoscopy, including push enteroscopy, is the primary diagnostic tool for upper GI bleeding [2, 4]. But repeated procedures or endoscopic ultrasound may be necessary if initial results are inconclusive. For obscure bleeding, invasive options like laparoscopy or laparotomy combined with push enteroscopy might be required [4]. Dieulafoy’s lesion is diagnosed by: normal mucosa around a small defect with pulsatile bleeding <3 mm; a protruding vessel from a slight defect or normal mucosa; and a fresh clot attached to a defect in normal mucosa [2].
Endoscopic treatments for Dieulafoy lesions include thermal (heat probe, argon plasma), injection (epinephrine, norepinephrine), and mechanical methods (banding, hemoclips), often combined for better outcomes [2]. If these methods fail, selective arterial embolization can be used as an alternative, involving angiography to identify the bleeding artery, which is then embolized with glue or coils [5]. Surgical intervention remains a final option with a high success rate for refractory cases [4]. The CT scan, in this case, confirmed a Dieulafoy’s lesion from the left phrenic artery, treated with embolization, and showed near-complete celiac trunk compression by the median arcuate ligament, treated conservatively. Median arcuate ligament syndrome (MALS) is a rare condition affecting 2 in 100 000, caused by celiac artery compression. It may have genetic and environmental causes and presents with bloating, weight loss, nausea, vomiting, and abdominal pain [6]. MRA and Doppler ultrasound are useful, but CTA is preferred for its detailed images, despite radiation and contrast risks [6]. MALS leads to significant hemodynamic changes, potentially causing pancreaticoduodenal aneurysms, splenic infarction, and splenic vein thrombosis due to reduced celiac artery flow. Severe compression can result in mesenteric ischemia and visceral infarction, with the spleen being especially vulnerable [7]. Treatment of MALS involves surgically decompressing the celiac artery. Minimally invasive methods generally result in faster recovery and less pain but have their risks. If stenosis persists, additional procedures like stenting may be necessary [6, 8].
Though both Dieulafoy’s lesion and MALS involve vascular anomalies, no established link exists between them. We suggest that celiac artery compression may affect other vascular lesions, but more research is needed to confirm this.
In this rare case, splenic infarction occurred as a complication following the embolization of the left phrenic artery to treat a Dieulafoy lesion. While embolization is effective for controlling gastrointestinal bleeding, it carries the risk of affecting nearby organs, such as the spleen, by altering blood flow or inadvertently occluding collateral vessels, leading to ischemia and infarction. Studies report splenic infarctions in 47.3% of patients treated in the splenic hilum compared to 12.5% in those treated in the main splenic artery [9], with distal embolization causing higher infarction rates (24%) than proximal embolization (6%) [10]. Additionally, up to 37.5% of patients with splenic infarction present with left-sided pleural effusion, which can arise due to sympathetic responses [11], compression of posterior lymphatics by the enlarged spleen, or filtration of hemorrhagic splenic fluid due to perisplenic inflammation [11, 12]. In this case, the pleural effusion resulted from splenic infarction as a secondary complication following the embolization.
In managing Dieulafoy lesions complicated by splenic infarction and pleural effusion, early involvement of interventional radiology (IR) is crucial for improving outcomes [13]. In this case, embolization of the left phrenic artery effectively controlled bleeding, demonstrating the importance of angiography and embolization for vascular anomalies not responsive to endoscopic treatment. When complications like splenic infarction and pleural effusion arise, IR can offer therapeutic options, such as CT-guided drainage and selective embolization. A multidisciplinary approach, including gastroenterologists, radiologists, and surgeons, is essential for tailoring treatment and preventing severe complications [13].
The patient had a good prognosis after treatment for Dieulafoy’s lesion, with no further bleeding on follow-up endoscopies. She was stable at discharge but requires follow-up with gastroscopy, CT scan, and ultrasound. Multidisciplinary care is crucial.
Conclusion
This case shows the complex links between Dieulafoy’s lesion, splenic infarction, and left pleural effusion. The patient’s challenges, despite multiple interventions, underscore the need for thorough, tailored treatment. Future research should focus on similar cases to improve diagnosis and treatment.
Author contributions
Qusay Abdoh (Conceptualization), Alaa Zayed, and Enas Samara (Writing—review & editing)
All authors contributed to the composition of the original draft and participated in the literature search.
Conflict of interest statement
No conflict of interest.
Funding
None declared.
Ethical approval
Ethical approval is exempt/waived at our institution.
Consent
The patient provided written consent for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal upon request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Guarantor
Enas Samara.
References
Wilkins T, Khan N, Nabh A, Schade RR. Diagnosis and management of upper gastrointestinal bleeding.
Al-Busaidi, Alsalt, et al. Upper gastrointestinal bleeding due to Dieulafoy's lesion of the stomach: a rare case report.



