-
PDF
- Split View
-
Views
-
Cite
Cite
José Luis Acha Sánchez, Jhon E Bocanegra-Becerra, Luis Contreras Montenegro, Manuel Cueva, Adriana Bellido, Shamir Contreras, Oscar Santos, Microsurgery for basilar apex aneurysms: a case series, Journal of Surgical Case Reports, Volume 2024, Issue 11, November 2024, rjae720, https://doi.org/10.1093/jscr/rjae720
Close - Share Icon Share
Abstract
Although endovascular management has been increasingly adopted for basilar apex aneurysms (BAAs), microsurgery still represents an amenable treatment option. In this case series, six female patients (median age: 46 years) with six saccular high-riding BAAs (50% ruptured) were included. The median neck size was 5.3 mm (range: 2.9–7.9), and the median length from base to dome was 7.25 mm (range: 5.2–11.4). Preoperative complications included hydrocephalus (22%) and rebleeding (22%). All patients underwent clipping with a pterional craniotomy with extension into the temporal bone base. Intraoperative aneurysm rupture occurred in one patient (17%). Postoperative complications occurred in two patients (34%), of which one died because of extensive cerebral vasospasm and hospital-acquired pneumonia. At the 6-month follow-up, all remaining patients had modified Rankin scale scores ≤ 2. Microsurgery remains a viable option for BAAs in limited-resource settings. Technical success depends on delicate tissue work, in-depth anatomical knowledge, and maneuverability in narrow corridors.
Introduction
The basilar artery is a vital supplier of the posterior cerebral circulation [1]. It irrigates major neural structures in a compacted and deep-seated space, which reflects its confronting nature for surgical access [1]. Representing about 5–8% of cerebral aneurysms, basilar apex aneurysms (BAAs) are a life-threatening condition owing to the risk of rupture [2–4].
From the very early surgical experiences of Drs Olivecrona and Yasargil to the advent of endovascular techniques, treating BAAs has been a demanding endeavor [5]. In contemporary times, the optimal management of BAAs is evaluated in a case-by-case basis and considers the best patient interest [5–7]. Factors for treatment approach selection have involved the assessment of the procedure’s invasiveness and its potential for durable and less morbid outcomes [6, 7]. In fact, large clinical trials and single-center reviews have attempted to provide valuable knowledge for guiding treatment selection with varying degrees of technical success, aneurysm recurrence, and morbidity [2, 3, 8, 9].
In developed nations, decision-making usually includes consideration of endovascular and surgical modalities for treatment [1–7]. However, in developing countries, an overlooked factor has been the limited availability of endovascular devices [10]. In this respect, this study aims to exemplify a case series and discuss the neurological outcomes in a center mainly offering microsurgery for BAAs.
Methods
A retrospective analysis of clinical records of patients presenting to our institution between 2018 and 2023, who were treated by Dr. JLAS, was conducted. An Institutional Review Board approval was granted. Incomplete records or charts with unintelligible hand-written data were excluded. Aneurysms in the basilar artery located other than the apex were also excluded.
Relevant data were collected regarding the patients' demographic features, as well as their pre-, intra-, and post-operative courses.
Statistical analysis
Descriptive analysis was performed using IBM® SPSS® software (version 29). Categorical and continuous data were summarized as proportions and measures of central tendency, respectively. The final data report was processed according to the STROBE Statement.
Results
A total of six female patients with a median age of 46 years (range: 44–67) were included in our analysis (Table 1). The median Glasgow Coma Scale (GCS) score at presentation was 14. Among the presenting symptoms, headaches constituted the most prevalent symptom (44%). Six saccular high-riding BAAs (50% ruptured) were present and coexisted with two anterior circulation aneurysms (Table 1). The median neck size of BAAs was 5.3 mm (range: 2.9–7.9), and the median length from base to dome was 7.25 mm (range: 5.2–11.4). Subarachnoid hemorrhage was present in 83% of patients upon arrival, with a median estimated time from symptoms' onset to presentation of 3 days (range: 1–5). Details on patient baseline characteristics are presented in Table 1.
| N° . | Age . | Sex . | Comorbidity . | Clinical presentation . | Hunt & Hess Grading Score . | Fisher Grading Scale . | Aneurysm features . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Rupture status . | Location . | Size [neck/length] (mm) . | Morphology . | |||||||
| 1 | 58 | F | Hypertension | Acute HA, LOC | 4 | 4 | Rp | Basilar Apex | 6.3/8.7 | Sc |
| 2 | 44 | F | None | Acute HA | 1 | 3 | Rp | P1-P2 | 5.6/13.6 | Sc |
| UR | Basilar Apex | 2.9/3 | Sc | |||||||
| 3 | 46 | F | None | Chronic HA | UR | Basilar Apex | 7.9/11.4 | Sc | ||
| 4 | 45 | F | None | Acute HA, LOC | 2 | 4 | Rp | ACoA | 4.3/6.8 | Sc |
| UR | Basilar Apex | 3.5/5.2 | ||||||||
| 5 | 65 | F | None | HA, vomiting | 1 | 4 | Rp | Basilar Apex | 4.3/5.8 | Sc |
| 6 | 67 | F | None | Coma | 3 | 4 | Rp | Basilar Apex | 7.3/10.2 | Sc |
| N° . | Age . | Sex . | Comorbidity . | Clinical presentation . | Hunt & Hess Grading Score . | Fisher Grading Scale . | Aneurysm features . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Rupture status . | Location . | Size [neck/length] (mm) . | Morphology . | |||||||
| 1 | 58 | F | Hypertension | Acute HA, LOC | 4 | 4 | Rp | Basilar Apex | 6.3/8.7 | Sc |
| 2 | 44 | F | None | Acute HA | 1 | 3 | Rp | P1-P2 | 5.6/13.6 | Sc |
| UR | Basilar Apex | 2.9/3 | Sc | |||||||
| 3 | 46 | F | None | Chronic HA | UR | Basilar Apex | 7.9/11.4 | Sc | ||
| 4 | 45 | F | None | Acute HA, LOC | 2 | 4 | Rp | ACoA | 4.3/6.8 | Sc |
| UR | Basilar Apex | 3.5/5.2 | ||||||||
| 5 | 65 | F | None | HA, vomiting | 1 | 4 | Rp | Basilar Apex | 4.3/5.8 | Sc |
| 6 | 67 | F | None | Coma | 3 | 4 | Rp | Basilar Apex | 7.3/10.2 | Sc |
ACoA: anterior communicating artery. HA: headache. LOC: loss of consciousness. Rp: ruptured. Sc: saccular. UR: unruptured.
| N° . | Age . | Sex . | Comorbidity . | Clinical presentation . | Hunt & Hess Grading Score . | Fisher Grading Scale . | Aneurysm features . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Rupture status . | Location . | Size [neck/length] (mm) . | Morphology . | |||||||
| 1 | 58 | F | Hypertension | Acute HA, LOC | 4 | 4 | Rp | Basilar Apex | 6.3/8.7 | Sc |
| 2 | 44 | F | None | Acute HA | 1 | 3 | Rp | P1-P2 | 5.6/13.6 | Sc |
| UR | Basilar Apex | 2.9/3 | Sc | |||||||
| 3 | 46 | F | None | Chronic HA | UR | Basilar Apex | 7.9/11.4 | Sc | ||
| 4 | 45 | F | None | Acute HA, LOC | 2 | 4 | Rp | ACoA | 4.3/6.8 | Sc |
| UR | Basilar Apex | 3.5/5.2 | ||||||||
| 5 | 65 | F | None | HA, vomiting | 1 | 4 | Rp | Basilar Apex | 4.3/5.8 | Sc |
| 6 | 67 | F | None | Coma | 3 | 4 | Rp | Basilar Apex | 7.3/10.2 | Sc |
| N° . | Age . | Sex . | Comorbidity . | Clinical presentation . | Hunt & Hess Grading Score . | Fisher Grading Scale . | Aneurysm features . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Rupture status . | Location . | Size [neck/length] (mm) . | Morphology . | |||||||
| 1 | 58 | F | Hypertension | Acute HA, LOC | 4 | 4 | Rp | Basilar Apex | 6.3/8.7 | Sc |
| 2 | 44 | F | None | Acute HA | 1 | 3 | Rp | P1-P2 | 5.6/13.6 | Sc |
| UR | Basilar Apex | 2.9/3 | Sc | |||||||
| 3 | 46 | F | None | Chronic HA | UR | Basilar Apex | 7.9/11.4 | Sc | ||
| 4 | 45 | F | None | Acute HA, LOC | 2 | 4 | Rp | ACoA | 4.3/6.8 | Sc |
| UR | Basilar Apex | 3.5/5.2 | ||||||||
| 5 | 65 | F | None | HA, vomiting | 1 | 4 | Rp | Basilar Apex | 4.3/5.8 | Sc |
| 6 | 67 | F | None | Coma | 3 | 4 | Rp | Basilar Apex | 7.3/10.2 | Sc |
ACoA: anterior communicating artery. HA: headache. LOC: loss of consciousness. Rp: ruptured. Sc: saccular. UR: unruptured.
Preoperative complications included hydrocephalus (22%) and rebleeding (22%). All patients underwent microsurgical management with aneurysm clipping (Table 2). In all cases, a pterional craniotomy with extension into the temporal bone base was performed. Intraoperative aneurysm rupture occurred in only one patient (17%) and was promptly controlled. Postoperative complications occurred in two patients (34%), of which one died because of extensive cerebral vasospasm and hospital-acquired pneumonia. The remaining five patients had a median GCS score of 15 at hospital discharge. At the 1-month and 3-month follow-up, 83% of patients had modified Rankin scale scores (mRS) ≤ 2. At the 6-month follow-up, all patients had an mRS ≤ 2 (Table 2).
| Case N° . | Craniotomy . | Postoperative complications . | Hospital discharge . | mRS score during follow-up . | |||
|---|---|---|---|---|---|---|---|
| GCS score . | mRS score . | 1-month . | 3-month . | 6-month . | |||
| 1 | Pterionala | Pneumonia, Ischemic Stroke and Cerebral Vasospasm | 6 | ||||
| 2 | Pterionala | None | 15 | 1 | 1 | 1 | 0 |
| 3 | Pterionala | None | 15 | 1 | 1 | 1 | 0 |
| 4 | Pterionala | None | 15 | 1 | 1 | 1 | 0 |
| 5 | Pterionala | Hydrocephalus | 15 | 1 | 1 | 1 | 0 |
| 6 | Pterionala | None | 14 | 1 | 1 | 1 | 0 |
| Case N° . | Craniotomy . | Postoperative complications . | Hospital discharge . | mRS score during follow-up . | |||
|---|---|---|---|---|---|---|---|
| GCS score . | mRS score . | 1-month . | 3-month . | 6-month . | |||
| 1 | Pterionala | Pneumonia, Ischemic Stroke and Cerebral Vasospasm | 6 | ||||
| 2 | Pterionala | None | 15 | 1 | 1 | 1 | 0 |
| 3 | Pterionala | None | 15 | 1 | 1 | 1 | 0 |
| 4 | Pterionala | None | 15 | 1 | 1 | 1 | 0 |
| 5 | Pterionala | Hydrocephalus | 15 | 1 | 1 | 1 | 0 |
| 6 | Pterionala | None | 14 | 1 | 1 | 1 | 0 |
aWith extension to the base of the temporal bone. GCS: Glasgow Coma Scale; mRS: modified Rankin Scale.
| Case N° . | Craniotomy . | Postoperative complications . | Hospital discharge . | mRS score during follow-up . | |||
|---|---|---|---|---|---|---|---|
| GCS score . | mRS score . | 1-month . | 3-month . | 6-month . | |||
| 1 | Pterionala | Pneumonia, Ischemic Stroke and Cerebral Vasospasm | 6 | ||||
| 2 | Pterionala | None | 15 | 1 | 1 | 1 | 0 |
| 3 | Pterionala | None | 15 | 1 | 1 | 1 | 0 |
| 4 | Pterionala | None | 15 | 1 | 1 | 1 | 0 |
| 5 | Pterionala | Hydrocephalus | 15 | 1 | 1 | 1 | 0 |
| 6 | Pterionala | None | 14 | 1 | 1 | 1 | 0 |
| Case N° . | Craniotomy . | Postoperative complications . | Hospital discharge . | mRS score during follow-up . | |||
|---|---|---|---|---|---|---|---|
| GCS score . | mRS score . | 1-month . | 3-month . | 6-month . | |||
| 1 | Pterionala | Pneumonia, Ischemic Stroke and Cerebral Vasospasm | 6 | ||||
| 2 | Pterionala | None | 15 | 1 | 1 | 1 | 0 |
| 3 | Pterionala | None | 15 | 1 | 1 | 1 | 0 |
| 4 | Pterionala | None | 15 | 1 | 1 | 1 | 0 |
| 5 | Pterionala | Hydrocephalus | 15 | 1 | 1 | 1 | 0 |
| 6 | Pterionala | None | 14 | 1 | 1 | 1 | 0 |
aWith extension to the base of the temporal bone. GCS: Glasgow Coma Scale; mRS: modified Rankin Scale.
Illustrative cases
Case I (patient nº1)
A 58-year-old female presented to the emergency department with a one-day course of headaches and loss of consciousness (Table 1). Cerebral tomography angiography (CTA) and three-dimensional reconstruction imaging revealed a ruptured BAA of 8.7 × 6.3 mm with a bleb formation (Fig. 1A–C). In the preoperative course, she developed rebleeding and cerebral vasospasm. A right pterional craniotomy with extension into the temporal bone base was performed. After gentle dissection of the dura mater and opening of the Sylvian fissure, identification of landmark triangles (white: carotid-oculomotor, sky blue: optic carotid, and green: supra carotid-infrafrontal) permitted access to the surgical corridor and clip placement at the BAA neck (Fig. 1D–F). Intraoperative fluorescein angiography corroborated blood flow permeability in the posterior circulation (Fig. 1G). Her postoperative imaging reconstruction demonstrated adequate placement of the clip (Fig. 1H–I). She was transferred to the neurointensive care unit, where she developed cerebral vasospasm and pneumonia. Unfortunately, because of these complications, the patient died (Table 2).
![Preoperative CTA, surgical images, and postoperative imaging (illustrative case I). (A–C) Cerebral CTA and three-dimensional reconstruction imaging revealed a BAA of 8.7 × 6.3 mm (green arrows); (D) The operative field depicted the corridors along with the identification of landmark triangles (white: carotid-oculomotor, sky blue: optic carotid, and green: supra carotid-interfrontal); (E–F) access into the carotid-oculomotor triangle permitted access to the basilar aneurysm for clipping; (H–I) Postoperative three-dimensional imaging reconstruction demonstrated clip placement at the aneurysm neck. BAA: basilar apex aneurysm; CTA: computed tomography angiography. Reprinted from World Neurosurgery, Vol. 191, Bocanegra-Becerra JE, Acha Sánchez JL, Microsurgical Clipping of a Ruptured Basilar Apex Aneurysm: Contending with a Formidable Clinical Scenario, Pages 35–36, Copyright (2024), with permission from Elsevier [11].](https://oupdevcdn.silverchair-staging.com/oup/backfile/Content_public/Journal/jscr/2024/11/10.1093_jscr_rjae720/1/m_rjae720f1.jpeg?Expires=1768775943&Signature=Y0xZA0sRnajearjLFyNMpODS7UR4m5-~72M1Lk7Y30gdn3imU6oEYexyvSnl3W3CYQfO7ZlQq460dWLePrgWfqS2ZFuLWm2QOowMubjd1qXnoRDq3pI8Yf3PIPNUMWKmfq3Emaqm8xP19nynQ2oMU6TWjQC~HqEcht-jT9~H7FRVRB~LhDTJqkQ~ty9ogl2rcde3jIohMg5N6yzVGGXIuy1GcDA2ASjuavz14Aac~uZdgou83Y89ViOjPSwKqQdDpzH3t58Wp2j5rD95zthIjcPPHq5HlAJnz-8KsUnXira3etzIBQEw42KuKl2-ApYksE0TyNuXpCeHKwwrrv0pQw__&Key-Pair-Id=APKAIYYTVHKX7JZB5EAA)
Preoperative CTA, surgical images, and postoperative imaging (illustrative case I). (A–C) Cerebral CTA and three-dimensional reconstruction imaging revealed a BAA of 8.7 × 6.3 mm (green arrows); (D) The operative field depicted the corridors along with the identification of landmark triangles (white: carotid-oculomotor, sky blue: optic carotid, and green: supra carotid-interfrontal); (E–F) access into the carotid-oculomotor triangle permitted access to the basilar aneurysm for clipping; (H–I) Postoperative three-dimensional imaging reconstruction demonstrated clip placement at the aneurysm neck. BAA: basilar apex aneurysm; CTA: computed tomography angiography. Reprinted from World Neurosurgery, Vol. 191, Bocanegra-Becerra JE, Acha Sánchez JL, Microsurgical Clipping of a Ruptured Basilar Apex Aneurysm: Contending with a Formidable Clinical Scenario, Pages 35–36, Copyright (2024), with permission from Elsevier [11].
Case II (patient n°4)
A 45-year-old female presented to the emergency department with a two-day history of intense headaches and loss of consciousness. Brain imaging with CTA and three-dimensional reconstruction revealed a ruptured anterior communicating aneurysm and an unruptured BAA (Fig. 2A–E). During her preoperative course, the patient experienced complications such as hydrocephalus, cerebral edema, and rebleeding that prompted medical attention. The patient underwent microsurgical management of both aneurysms. A right pterional craniotomy with extension into the temporal bone base was performed. After gentle dissection of the dura mater and opening of the Sylvian fissure, identification of the carotid cistern and surrounding anatomy allowed for access into the corridor toward the BAA (Fig. 2F–H). The patient tolerated the procedure well, and her postoperative CT scan demonstrated adequate placement of clips over the aneurysm necks (Fig. 2I). The patient was discharged with a GCS score of 15. At the 6-month follow-up, the patient was neurologically intact.
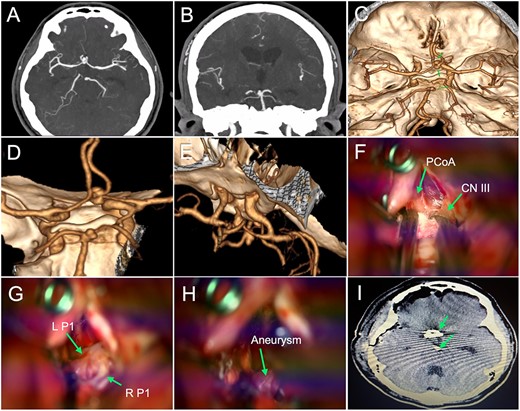
Preoperative imaging, surgical images, and postoperative tomography (illustrative case II). (A–B) CTA revealed saccular aneurysms located in the anterior communicating artery and BAA; (C–E) Three-dimensional image reconstruction showed a bilobed anterior communicating aneurysm of 4.3 × 6.8 mm and a saccular BAA of 3.5 × 5.2 mm (green arrows); (F–H) The operative field depicted the corridors along with major structures (i.e. PCoA: posterior communicating artery, CN III: cranial nerve III, L P1: left P1 segment, and R P1: right P1 segment) that were carefully handled to access the BAA (green arrows); (I) Postoperative head tomography showed the placement of clips in the aneurysm necks (green arrows). BAA: basilar apex aneurysm; CTA: computed tomography angiography.
Discussion
Microsurgical management of BAAs demands delicate tissue disruption, thorough anatomical knowledge, and maneuverability in narrow corridors [6, 7]. On the other hand, endovascular treatment represents a less invasive method to occlude the aneurysmal sac through the placement of coils, stents, or flow diverters [4, 6, 7]. It is worth noting that limited outcomes regarding treatment superiority have been reported in landmark trials. For instance, the International Subarachnoid Aneurysm Trial (ISAT) and the International Study of Unruptured Intracranial Aneurysms (ISUIA) trial compared a scant number of BAAs treated with surgery vs endovascular treatment, with a prevalence of only 0.8% and 1.7%, respectively [2, 5, 8, 9]. In the latter trial, clinical outcomes were similar between treatments for BAAs ≤12 mm [2, 5]. Regarding complete/near complete occlusion, large case series have reported rates of 59–89% and 66–98% for the endovascular and microsurgical treatment, respectively [12–26]. In a systematic review comparing both interventions, the weighted average favorable clinical outcome was significantly higher in the endovascular group (86.4 vs 79.6%, P < 0.0001), while the weighted average complete/near complete occlusion rate was significantly higher in the surgical group (92.6 vs 83.8%, P < 0.0001) [6].
Although, BAAs are more likely to be treated endovascularly in recent years, microsurgical management is still amenable for aneurysms with unfavorable morphology (i.e. large/giant size, wide necks, irregular shapes, or poor aspect ratios) [20, 24]. There are various surgical approaches that have been described to achieve aneurysm occlusion. Each approach has its own advantages and limitations [1]. Some approaches include the transsylvian, orbitozygomatic, pretemporal transzygomatic transcavernous, pterional approach through the extended lateral corridor, lateral supraorbital and subtemporal approaches [27]. Factors accounting for selecting the correct approach include and are not limited to the aneurysm projection, the relationship of parent arteries with the origin of P1 segments, and the configuration of perforators [20].
Our case series was performed in an institution with limited availability of endovascular devices. In this context, surgical management is the predominant treatment choice for optimizing the clinical course of patients. In addition, a major hurdle in treating these patients is the late arrival to our facilities, as exemplified by an estimated median time of symptoms' onset to arrival of 3 days. Although the analysis of factors contributing to this late presentation is beyond the scope of this study, the lack of access to neurosurgical care in places outside the country's capital could be a contributing factor, as 50% of patients resided in provinces. Moreover, the burdensome course of BAAs further contributed to the mortality rate [11].
Our study findings suggest that microsurgical treatment of BAAs—in experienced hands—results effective in providing good clinical neurological outcomes, which coincide with outcomes from large case series [6, 19, 21, 22, 28]. An important perspective on case selection, given the constraints of resource availability, was not about choosing between microsurgery and endovascular treatment; rather, it focused on the expertise of the surgical team in operating on BAAs.
Study limitations
Our case series is subject to several limitations that can restrict its external validity. As a single-center experience involving a limited number of cases in a resource-constrained facility, conclusions might not be extrapolated to standard surgical practice. Another noticeable limitation was the retrospective approach to clinical records. Furthermore, postoperative outcomes were only available for review for up to 6 months, which limits the comprehensive assessment of treatment durability.
Conclusion
In the contemporary era, microsurgery remains viable for BAAs, especially in settings where endovascular management is not present in the decision-making algorithm. Key determinants of technical success and good neurological outcomes are intuitively linked to surgical expertise with delicate tissue handling, in-depth anatomical knowledge, and maneuverability in narrow corridors.
Conflict of interest statement
None declared.
Funding
None declared.
Data availability
Some of the clinical information presented in this study is derived from a larger dataset that our research group has published previously [10].
Ethical statement
IRB approval was granted.



