-
PDF
- Split View
-
Views
-
Cite
Cite
Rebecca B J Cui, Susan Hawes, Alison J Potter, Kathleen Merrick, Sanjay Warrier, Farhad Azimi, Dermatofibrosarcoma protuberans of the breast in pregnancy, Journal of Surgical Case Reports, Volume 2024, Issue 1, January 2024, rjad738, https://doi.org/10.1093/jscr/rjad738
Close - Share Icon Share
Abstract
Dermatofibrosarcoma Protuberans (DFSP) is a rare, locally aggressive fibroblastic mesenchymal neoplasm, typically derived from the dermis, with the intramammary subtype being seen infrequently. We present a case of a 40-year-old woman whom was diagnosed with an intramammary DFSP during pregnancy, whom underwent successful surgical management during her second trimester. Our case demonstrates the importance of increased clinical awareness in the diagnosis and treatment of breast DFSP with careful multidisciplinary consideration.
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare, locally aggressive fibroblastic mesenchymal neoplasm, typically derived from the dermis [1, 2]. Involvement of the breast is infrequent and usually presents as an exophytic breast mass of the dermis, with the intramammary subtype of breast DFSPs being more uncommon [3].
Case report
A 40-year-old pregnant female at 8-weeks gestation had an incidental finding of a left breast lesion on investigation for contralateral mastalgia. A mammogram was unremarkable, whilst ultrasound showed a 13 × 9 × 10 mm irregular echogenic lesion with internal vascularity that was within intramammary fat, without connection to the dermal plane (Fig. 1). There were no palpable masses or lymphadenopathy on examination. The patient was otherwise well, with no family history of breast, ovarian cancer or sarcoma.
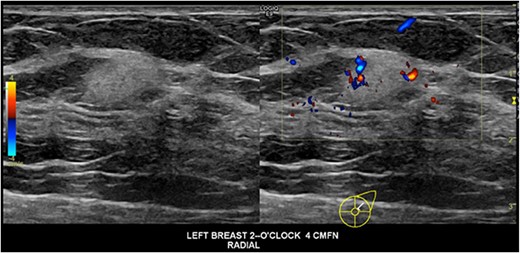
Ultrasound images of the lesion demonstrating an irregular hyperechoic lesion with internal vascularity.
Core biopsy of the lesion showed a cellular spindle cell lesion with buckled spindled cells arranged in storiform swirls featuring eosinophilic cytoplasm, and bland tapered nuclei with uniform chromatin. Immunohistochemical panel showed the cells were positive for CD34, and negative for SMA, desmin, EMA, STAT6, S100, SOX10, ALK-1, PanTRK, ER, PR, AR, and panCK. Fluorescence in-situ hybridisation testing for COL1A1-Platelet Derived Growth Factor-β (COL1A1-PDGFβ) fusion product rearrangement was negative, and further ribonucleic acid (RNA) fusion panel testing did not identify any chromosomal deletions. These findings were concerning for DFSP.
Decision was made for wide local excision with 2 cm margins with excision of an overlying ellipse of skin after discussion at the sarcoma multidisciplinary team meeting. Interestingly, as the pregnancy progressed, repeat ultrasound showed a slight decrease in tumour size. Surgery was performed when the patient reached 16-weeks gestation, given the second trimester is the safest period to undergo general anaesthetic. The lesion and overlying skin were excised with hookwire-guidance and primarily closed. There were no post-operative complications (Fig. 2).
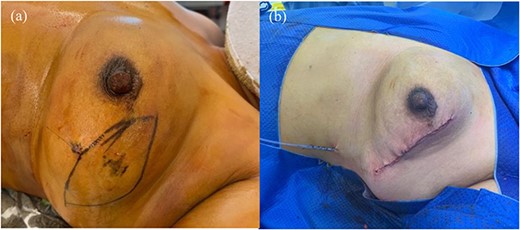
Operative images, (a) Pre-operative marking of excision margin with wire in-situ. (b) Wound following wide local excision of the tumour with ellipse of skin and primary closure.
Histopathology showed a 10 × 10 mm tumour composed of bland and crumpled spindle cells with a storiform arrangement showing a honeycomb pattern of infiltration into the surrounding adipose tissue (Fig. 3). There was minimal mitotic activity and only mild cytological atypia. The lesion had strong expression of CD34 whilst a range of keratin stains (HMWCK, CAM 5.2, CK 5/6, CK14, and AE1/AE3) were negative. There was no involvement of the skin seen. This is consistent with a DFSP of the breast, with intramammary subtype. The excision margins were clear, with the closest being 12 mm from the superficial margin, and ˃20 mm from all other margins. There was a background of widespread secretory and lactational change of the breast consistent with pregnancy, and an incidental single focus of atypical ductal hyperplasia seen.
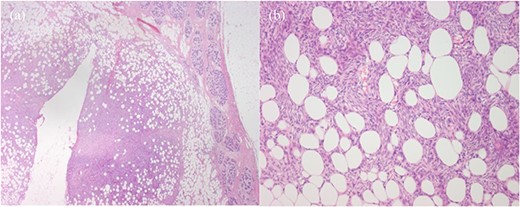
Histopathology, (a) Haematoxylin and eosin (H&E)-stained section of breast parenchyma showing lactational change and containing an ill-defined spindled cell tumour and a central defect from localisation wire. (b) H&E section of tumour with bland spindled cells in tight storiform arrays that infiltrate and entrap adjacent adipocytes (fat), producing a honeycomb pattern.
Discussion
Given the rarity of intramammary breast DFSPs, they may be mistaken for fibroadenomas or other benign breast masses on imaging. On ultrasound they are usually distinct heterogeneously hyperechoic with hypervascularity on doppler, as seen in our patient. On mammogram these lesions are usually mammographically dense without calcifications or fatty components [3]. Magnetic resonance imaging may be useful with diagnosis, where the lesion will be hypointense to isointense on T1, hyperintense on T2, and rapidly enhancing with contrast, or may mimic fibroadenoma or intramammary lymph node [4].
Diagnosis with core biopsy is crucial as histopathologically DFSPs have a characteristic appearance with storiform architecture, infiltrative ‘honeycomb’ growth within subcutaneous adipose tissue, and immunoreactivity for CD34. In total, ˃90% of cases are associated with translocation of COL1A1-PDGFβ fusion product. A variant mutation of COL6A3-PDGFD fusion product has been shown to have a predilection for breast DFSPs [1, 5], however detection of this translocation is limited in the clinical setting.
The treatment for DFSP of the breast is primarily surgical, with wide local excision of the lesion of at least 2 cm margins. Surgical techniques described include wide local excision with primary closure, or reconstructive procedures with flaps or skin grafts, and Mohs micrographic surgery to ensure adequate margin control [6]. Furthermore, DFSP is a radio- responsive tumour, where adjuvant radiotherapy is used for recurrent or unresectable cases.
Tyrosine Kinase therapy (Imatinib) may be warranted in tumours with COL1A1-PDGFβ fusion gene [2].
In the literature, the effect of pregnancy on DFSP shows potential associated enlargement and aggressive behaviour, mediated by PDGF’s role in embryogenesis and increase in progesterone hormones [7–10]. Only one case of breast DFSP in pregnancy has been reported in which the patient had a rapidly growing breast mass [11].
Conclusion
Our case demonstrates the importance of increased clinical awareness in the diagnosis and treatment breast DFSP. Management of a breast DFSP in pregnancy has further considerations including the risk of general anaesthetic on the foetus, and the effects of breast surgery on future breast-feeding. Though rare, the literature suggests a possible association with tumour enlargement during pregnancy, and the paucity of literature in this population warrants careful multidisciplinary consideration with the management of these patients.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
None declared.



