-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Kent, David Saulino, Incidental multifocal granular cell tumor in the setting of chronic gastritis discovered following gastric sleeve operation: a case report and brief review of the literature, Journal of Surgical Case Reports, Volume 2024, Issue 1, January 2024, rjad700, https://doi.org/10.1093/jscr/rjad700
Close - Share Icon Share
Abstract
Granular cell tumors are uncommon neoplasms of Schwannian origin that can present nearly anywhere in the body, but are most commonly seen in the head and neck region. While the majority of these tumors are benign, a subset can behave aggressively and even have metastatic potential. We are reporting a case of a 35-year-old female with a history morbid obesity presenting for bariatric surgery (sleeve gastrectomy). Upon receiving the specimen in pathology, nodular vaguely defined lesions were identified in the gastric wall. Representative sections were submitted for microscopic examination, which revealed the incidental finding of a multifocal granular cell tumor with a background of chronic gastritis. The patient has not had any regional recurrences or metastasis in the past 2 years following the procedure.
Introduction
Granular cell tumors (GCT) are an uncommon type of peripheral nerve sheath tumor of Schwannian origin that can present in widely variable anatomic locations [1, 2]. GCTs most commonly present in the head and neck region, digestive tract, or skin/subcutaneous tissue [3]. GCTs are most frequent in the fourth to sixth decades, although there are many exceptions including pediatric and congenital cases [4–6]. Gender predilection is still a debatable matter depending on the study that is cited, although one very large study reported a male predominance for patient presenting with this tumor [4, 7]. Gross examination of these tumors typically reveals a gray-white to pale-yellow cut surface [4]. These lesions usually show sheets to cord-like growth of cells with abundant eosinophilic granular cytoplasm upon light microscopic examination; when immediately under the skin, this tumor is known to induce pseudoepitheliomatous growth of the overlying epithelium that can even mimic squamous cell carcinoma [4]. A nearly universal distinguishing histologic characteristic of GCTs is the pustule-ovoid bodies of Milian (POBoM), which appear as a pale halo surrounding the eosinophilic bodies [4]. POBoM are thought to result from a progressive accumulation of granules within lysozymes [8]. As a result, it could be postulated that more established tumors could have an increased number of POBoM. GCT is almost always positive for S-100 immunohistochemical staining (IHC). Other markers that can be used include CD56, PGP9.5, p75, and neuron-specific enolase. GCTs should be negative for GFAP, neurofilament marker, chromogranin A, and synaptophysin [4]. Most GCT are benign lesions without metastatic potential, but a subset of these tumors can behave in a frankly malignant fashion (~0.5%–2.0%) [9]. Unfortunately, predicting behavior in any given lesion can be challenging, as even GCTs with a benign appearance can progress to metastatic disease in rare instances [4]. In general, increased mitoses, an elevated Ki-67 proliferative index (>10%), aberrant P53 IHC expression, and necrosis are features more likely to be associated with aggressive behavior [4, 10, 11]. Large tumor size may also be an adverse prognostic factor [11, 12]
Case report
A 35-year-old female presented to the hospital for a gastric sleeve operation given her history of morbid obesity (BMI 50.0–59.9). The sleeve gastrectomy specimen was received into pathology and measured 22.5 × 4 × 1.5 cm and contained a single, stapled margin. Upon sectioning the specimen, a 0.8 × 0.4 × 0.4 cm firm white nodule with indistinct borders was identified 2.9 cm from the stapled margin. Further sectioning revealed two additional mural nodules of similar coloration measuring 0.6 and 0.4 cm, respectively, with each being located 2.5 cm from the stapled margin. All the lesions were submitted in their entirety for microscopic examination in addition to routine sections of the background gastric tissue. Examination of the lesional sections under light microscopy revealed these proliferations to be deep in the submucosa with tumor cells abutting the inner band of the muscularis propria (Fig. 1). The tumor was predominately composed of individual or small nests of tumor cells enmeshed in a collagenous stroma. The cells displayed abundant eosinophilic granular cytoplasm with indistinct cell borders. Scattered intracytoplasmic spherical bodies surrounding by a clear halo (POBoM) were noted (Fig. 1). The tumor nuclei were variable in size, round to oval, with some showing small nucleoli and mild nuclear membrane irregularity. No significant mitotic activity or necrosis was noted in any of the tumor sections. S-100 IHC staining demonstrated diffuse nuclear positive staining in the tumor cells (Fig. 1). Sections of the background gastric mucosa were remarkable for moderate lymphoplasmacytic inflammation and frequent lymphoid aggregates. Helicobacter Pylori IHC was negative. Chromogranin IHC was also performed to assess for neuroendocrine cell hyperplasia and the possibility of autoimmune metaplastic atrophic gastritis (AMAG). Chromogranin did highlight scattered neuroendocrine cells but did not show linear or nodular neuroendocrine cell hyperplasia that would be supportive of AMAG; but the possibility of early/subclinical disease was suggested as something that could not be entirely excluded.
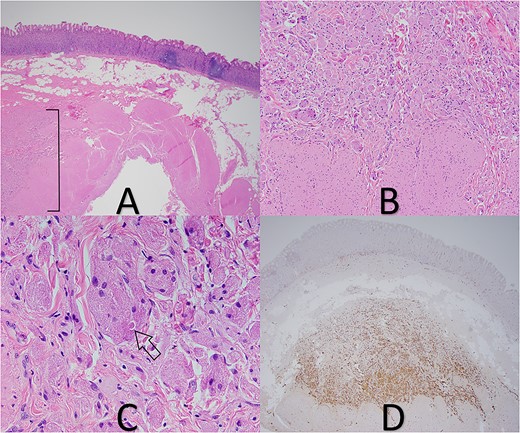
(A) GCT in deep submucosa with overlying gastric mucosa and adjacent chronic gastritis (tumor in bracket), 20× magnification. (B) Tumor cells abutting muscularis propria, 100× magnification. (C) Pustule-ovoid body of Milian (arrow), 400× magnification. (D) S-100 IHC stain highlights tumor cells, 20× magnification.
Discussion
While it is well known that GCT can present in nearly any anatomic location, gastric tumors are quite rare. In fact, a large study of 263 cases of GCT did not mention the stomach as a location at all, and also mentioned that multifocal tumors are uncommon [7]. Another study noted that gastric GCT only represented a small subset (4%) of the 98 tumors they assessed [13]. To the best of our knowledge, gastric GCT reported in the literature have had a universally indolent clinical course without reports of metastasis [13–16].
This particular case is notable as the tumor was not discovered until after surgery was performed for another indication. The patient in question has not had any recurrences in over 2 years since her initial operation, in keeping with the benign histologic features of her lesions. In our own institutional experience, we have also had a recent case of gastrointestinal stromal tumor being incidentally discovered during a sleeve gastrectomy, and have discovered multiple Helicobacter Pylori infections in such samples; giving strong justification for continuing to examine these specimens post resection even if overt abnormalities are not noted during the operative procedure.
The degree of chronic inflammation in the background gastric mucosa for this patient was unexpected and of uncertain clinical significance given the negative Helicobacter Pylori IHC. Follow-up stool antigen testing for Helicobacter Pylori was also negative. The possibility of early AMAG was mentioned in the pathology report and also considered clinically; anti-parietal cell antibody testing was equivocal, but anti-intrinsic factor antibody testing was negative and fasting serum gastrin levels were found to be in normal range.
Although work has been done to stratify GCT for risk assessment, it still remains somewhat challenging to predict behavior in these neoplasms. As more of these neoplasms are reported in the literature, especially atypical and malignant examples, it would be ideal if a molecular profile emerges that can help clinical providers to better anticipate recurrence and metastasis.
Conflict of interest statement
None declared.
Funding
None declared.



