-
PDF
- Split View
-
Views
-
Cite
Cite
Jesús Elías Ortiz Gómez, Mario Toledo Barranco, Willberto Medina Aguirre, José Aldo Guzmán Barba, Isaac Esparza Estrada, Patricia Ruiz Cota, José Oscar Orozco Álvarez Malo, Management of gastropleural leak by video-assisted thoracoscopy after sleeve gastrectomy, Journal of Surgical Case Reports, Volume 2023, Issue 8, August 2023, rjad479, https://doi.org/10.1093/jscr/rjad479
Close - Share Icon Share
Abstract
Obesity is a major public health issue with various comorbidities. Sleeve gastrectomy has become a popular treatment for obesity, but it carries the risk of complications, particularly staple line leakage. This case study focused on a 32-year-old woman with grade II obesity and hiatal hernia who underwent laparoscopic sleeve gastrectomy. Four days after surgery, she presented with abdominal pain, revealing leakage from the gastric sleeve into the thoracic cavity. Diagnostic procedures and interventions were performed, including cavity lavage, drainage placement, and stent placement. The patient showed clinical improvement after video-assisted thoracoscopic surgery and a multidisciplinary approach involving nutrition support and antibiotics. Despite the challenges, the patient’s clinical course improved, leading to discharge with no evidence of leakage on follow-up endoscopy. Careful monitoring and timely interventions are essential to manage complications in sleeve gastrectomy procedures and ensure optimal patient outcomes.
INTRODUCTION
Due to the various accompanying comorbidities, obesity, a global epidemic that has progressively spread over the past few decades, is currently one of the biggest public health issues. The World Health Organization (WHO) estimates that 13% of the total adult population is overweight or obese to some extent [1]. Extra body weight is linked to a higher risk of coronary artery disease, stroke, and death, as well as an increased risk of cardiometabolic diseases such as type 2 diabetes mellitus, hypertension, and dyslipidemia [2].
Over the past decade, the popularity of the weight-loss procedure known as sleeve gastrectomy has increased, making it one of the accepted treatments for obese individuals. The procedure is a minimally invasive laparoscopic surgery in which the portion of the stomach responsible for appetite control is removed [3]. The unique physiology of a sleeved stomach, which includes a high-pressure system from an intact pylorus distally and lower esophageal sphincter proximally, increases the likelihood of chronic leakage or fistulation to nearby anatomical compartments. Other etiologies and causes include iatrogenic injuries, inadequate vascularization, ischemia, hematoma formation, technical issues, and staple misfiring [4, 5].
However, these surgeries are not without complications, and one of the most severe side effects is the staple line leak. The reported rates range from 2 to 5%. In many cases, an early contrast material swallow test is not diagnostic, and the patient is discharged. The presence of gastric material collected subphrenically leads to thoracic and abdominal reactions. The formation of a gastro-pulmonary fistula is facilitated by the omental ‘walling off’ response, which inhibits disseminated peritonitis and leads to left pleural thickening, effusion, and adhesion between the left lower lobe and the diaphragm [6].
CASE REPORT
Thirty-two-year-old female with a diagnosis of grade II obesity (BMI 35.1) and hiatal hernia, without any other significant medical history, underwent laparoscopic sleeve gastrectomy + hiatal plasty on 08 January August 2021 (Fig. 1). The surgery was performed without apparent complications. During the early postoperative hours, the patient experienced nausea and vomiting on multiple occasions, which subsided with the prescribed medication. She was discharged 2 days later.
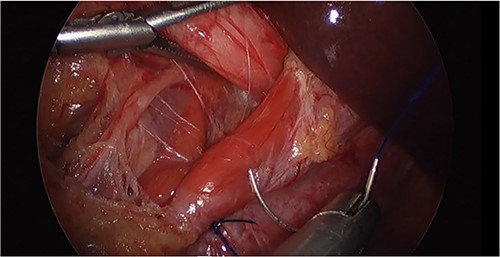
Laparoscopic image of the hiatal hernioplasty during the initial surgery.
Four days after surgery (12 January 2021), the patient returned due to unrelenting abdominal pain. Thoracic and abdominopelvic tomography was performed, revealing left-sided hydrothorax and leakage from the gastric sleeve into the thoracic cavity. Diagnostic laparoscopy was carried out, but no abdominal leakage site was identified. Additionally, migration of the sleeve through the hiatus was observed, despite the hernioplasty performed during the initial intervention (Fig. 2). Lavage of the cavity, and left pleural drainage were performed.
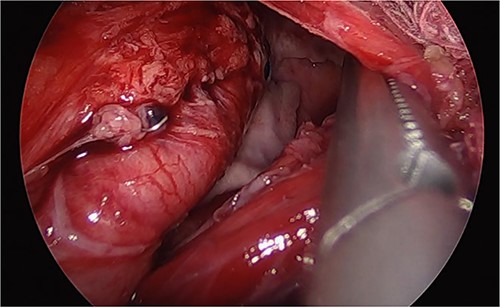
Laparoscopic image of the second surgery showing the presence of purulent fluid, fibrinopurulent tissue, and migration of the gastric sleeve into the thorax.
The following day (13 January 2021), an endoscopy was performed, revealing a leakage site at the mid-third of the gastric sleeve. A 12 cm esophagogastric stent was placed ~3–4 cm above the gastroesophageal junction (Fig. 3).
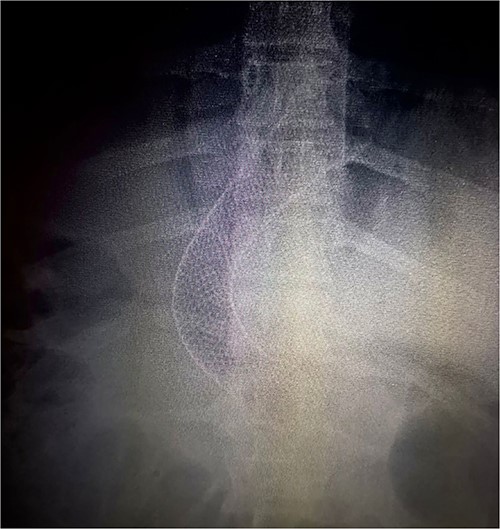
Thorax X-ray showing the placement of the gastroesophageal stent above the gastroesophageal junction.
On 19 January 2021, a tomographic control showed leakage from the esophagogastric junction and left-sided pneumonia (Figs 4 and 5). Consultation with the cardiothoracic surgery department led to the decision to perform VATS.
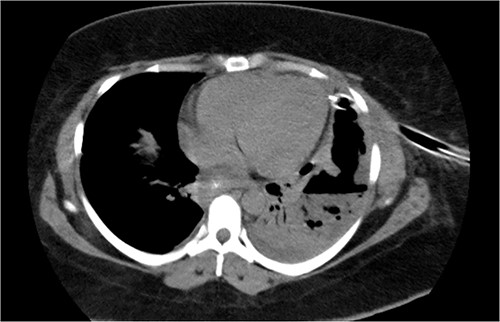
Chest tomography revealing the presence of left pleural fluid and cavitations.
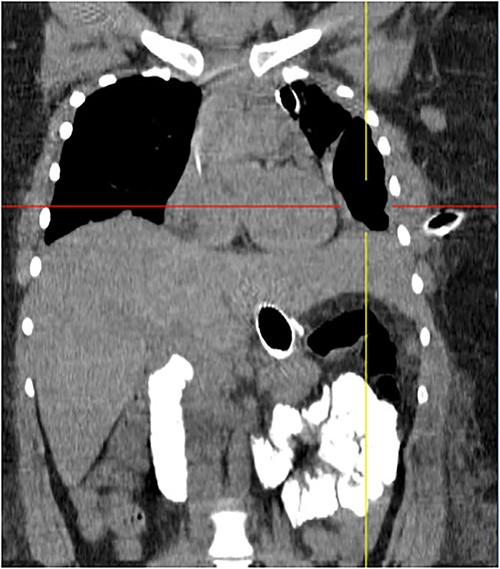
Coronal chest tomography showing the presence of the gastroesophageal stent and the left pleurothoracic cavity.
VATS was performed on 20 January 2021 for thoracic cavity lavage and placement of pleural drains (Fig. 6), along with abdominal laparoscopic revision and endoscopy for stent repositioning and nasojejunal tube placement. The patient showed clinical improvement after the procedure and with total parenteral nutrition support, enteral diet through the nasojejunal tube, broad-spectrum antibiotics guided by culture, and blood component transfusion.
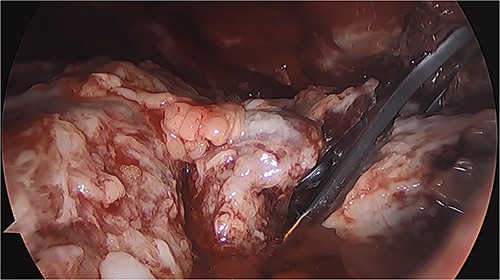
Thoracoscopy (VATS) revealing the presence of fibrinopurulent tissue in the left thoracic cavity.
Seven days later (27 January 2021), a leak test was performed, yielding negative results. Due to the patient’s favorable clinical course and controlled leakage, discharge was decided after 15 days of hospitalization, with continued follow-up at the original facility. A follow-up endoscopy was performed 2 months after surgery (22 March 2021), with a normal report and no evidence of leakage.
DISCUSSION
This complication most commonly occurs at the upper stapler line, near the gastroesophageal junction in Laparoscopic Sleeve Gastrectomy (LSG), if not identified and treated quickly and aggressively, it may lead to abdominal sepsis, occasionally progressing to multiorgan failure and death [7].
According to the Clinical Issues Committee of the American Society for Metabolic and Bariatric Surgery, the overall complication rate for LSG is 0–24%, and the mortality rate is 0.39%. Leaks following LSG happen in 1.4–5.3% of cases [8].
The presence of gastric sleeve-derived fistulas has been associated with patient-specific conditions such as nutritional status, BMI, use of immunosuppressive medications, chronic diseases that compromise functional reserve and healing, as well as conditions that increase intraluminal pressure, such as postoperative nausea and vomiting, rapid progression to solid foods, and distal obstruction [9].
Diagnosis can be difficult as the patients can present with nonspecific symptoms such as productive cough, shortness of breath, chest pain, or abdominal pain. Due to the variable symptoms, CT imaging and MRIs are at the forefront of diagnosis [10]. In this case, timely imaging studies prompted by the patient’s symptoms were a crucial pillar in diagnosing and treating this complication.
VATS is a minimally invasive procedure that enables proper lavage and drainage of the pleural cavity, effectively controlling infection and managing the filtered fluid originating from the leakage [11]. In our clinical case, attempts to resolve the leakage through the placement of an esophagogastric stent did not yield the expected result. Consequently, VATS was performed, successfully achieving resolution of the leakage.
Guidelines for the management of gastropleural leaks are limited. Certain authors advocate for a laparoscopic strategy, whereas others propose a conservative approach involving the use of antibiotics and percutaneous drainage [12]. A step-up approach for treatment was described by Shoar et al. [13] in which initially, noninvasive measures are employed, followed by minimally invasive procedures, and ultimately surgical intervention.
The timely diagnosis and classification of leaks are crucial. The treatment strategy depends on the patient’s clinical condition. We emphasize three main pillars: medical management, leak drainage, and defect repair. Medical management involves the early use of intravenous antibiotics, intravenous hydration, and nutrition. The assessment of the patient’s clinical stability will guide further management. Regarding surgical management, a minimally invasive approach should be chosen due to the high perioperative morbidity associated with surgery. A multidisciplinary approach is essential for managing these complex patients.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and analyzed during the current study are available upon reasonable request. Researchers interested in accessing the data may contact the corresponding author for further information and to request the datasets.
References
- antibiotics
- obesity
- stents
- abdominal pain
- endoscopy
- comorbidity
- diagnostic techniques and procedures
- follow-up
- hernia, hiatal
- irrigation
- nutritional support
- surgical procedures, operative
- thoracic surgery, video-assisted
- public health medicine
- sleeve gastrectomy, laparoscopic
- thoracic cavity
- patient-focused outcomes
- video assisted thoracoscopy



