-
PDF
- Split View
-
Views
-
Cite
Cite
Jae Han Kim, Seung Hyun Yoon, Ji Ahn Choi, Ji Hyeon Kwak, Milim Kim, Sung Hyun Kim, Pathologically confirmed pancreatic hamartoma after surgical resection with an aldosterone-producing adrenal tumor: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 8, August 2023, rjad475, https://doi.org/10.1093/jscr/rjad475
Close - Share Icon Share
Abstract
Pancreatic hamartoma is a benign tumor of the pancreas with an extremely low incidence and is commonly diagnosed by pathologic examination after surgery. This report describes the case of a 57-year-old female who was referred for the evaluation of a pancreatic mass and an adrenal incidentaloma. Further imaging studies suggested pancreatic neuroendocrine tumor and aldosterone-producing adrenal tumor. Pylorus-preserving pancreaticoduodenectomy was performed with the initial impression of a pancreatic neuroendocrine tumor. However, pathology results revealed a pancreatic hamartoma. Multiple endocrine neoplasia type 1 syndrome was discussed as a probable explanation for tumor masses in both the pancreas and adrenal gland.
INTRODUCTION
Hamartoma is a benign and tumor-like disorganized mass that consists of tissue elements normally found in the affected area [1]. The global incidence of hamartoma is unknown, possibly because of its rarity. While hamartoma can occur in various places of the body, the lung has been reported to be the most common site of hamartoma, with an incidence estimated to be 0.25% in the general population [2].
Thus, pancreatic hamartomas are expected to account for a small portion of hamartoma cases. To date, fewer than 100 cases of pancreatic hamartoma have been reported in the English literature [3, 4]. Because of its extremely low incidence and nonspecific findings in imaging studies such as computed tomography (CT), distinguishing between a hamartoma and a low-grade malignant mass in the pancreas has been challenging [5, 6].
In this case report, we presented a patient with tumor masses in both the pancreas and adrenal gland, which were suspected to be a pancreatic neuroendocrine tumor and an aldosterone-producing adenoma, respectively. The patient underwent pylorus-preserving pancreatoduodenectomy (PPPD) for pancreatic mass, and the pathology results confirmed it to be a pancreatic hamartoma.
CASE REPORT
A 57-year-old female was referred to the outpatient department of our institute since an enhanced abdominopelvic CT conducted in another hospital showed (i) a 3.3-cm-sized hypervascular mass with internal cystic changes in the uncinate process of the pancreas and (ii) a 1.6-cm-sized enhancing fat-containing lesion in the right adrenal gland (Fig. 1a and b). At the initial outpatient consultation, the patient denied any specific symptoms. She had no noteworthy medical history except hypertension and dyslipidemia requiring medication. Her family history was notable for pancreatic cancer in her father and sister, gastric cancer in her brother, and type 2 diabetes mellitus in her mother.
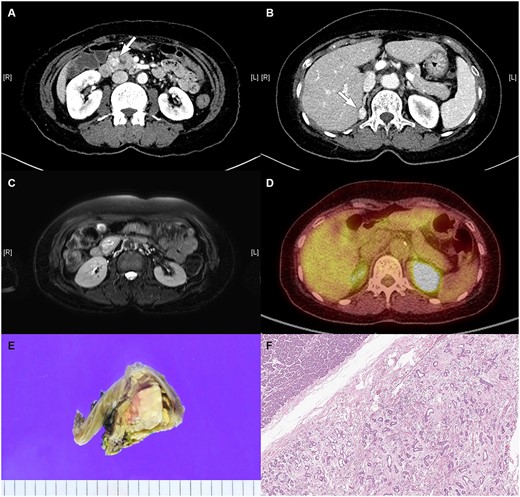
Radiologic images of the (A) pancreatic mass in the uncinate process of the pancreas on CT, (B) right adrenal mass on CT, (C) pancreatic mass on MRI, and (D) pancreatic mass on PET. (E) Gross pathology: well-demarcated whitish to tan-colored mass with mild hemorrhage and (F) microscopic findings: variably cystic small to medium-sized ductular structures surrounded by collagenized stroma.
To obtain more information on both masses, several laboratory and imaging studies were performed. Hormonal evaluation results of elevated aldosterone (399.38 pg/mL [reference: 37.82–388]) and low renin (<0.07 ng/mL/h [reference: 0.30–4.88]) levels suggested primary hyperaldosteronism because of an adrenal mass, although she experienced no specific symptoms. The patient’s sodium (144 mmol/L [reference: 135–145]) and potassium (3.6 mmol/L [reference: 3.5–5.5]) levels supported this impression, although they were within the normal ranges.
Magnetic resonance imaging (MRI) confirmed a 3.3-cm-sized hypervascular mass in the pancreas uncinate process and a 1.6-cm-sized enhancing fat-containing lesion in the right adrenal gland, corroborating the CT findings (Fig. 1c). PET-CT revealed increased fluorodeoxyglucose uptake with equivocal significance for malignancy between the pancreas head and duodenum and focal increased uptake in the right adrenal gland (Fig. 1d).
Accordingly, PPPD was planned with the impression of a pancreatic neuroendocrine tumor with cystic changes. The right adrenal mass was deemed unsuitable for adrenalectomy because of its size < 4 cm, below the criteria for surgical treatment [7]. Further evaluation of the right adrenal gland was postponed until after the surgery with consent of the patient, and she was prescribed spironolactone (Spirodactone) at a dose of 12.5 mg twice daily for hyperaldosteronism.
During the operation, the pancreas was soft, and the surgical specimen revealed a well-defined whitish to tan-colored mass (3.2 cm) with slight hemorrhage (Fig. 1e). The pathological examination showed pancreatic hamartoma. Microscopic findings showed a well-demarcated mass with an adenosis-like structure composed of small to medium-sized ducts with stromal fibrosis (Fig. 1f). Immunohistochemical staining demonstrated positive staining for CK7 and CK19 and focal positivity of the S-100 protein in ductular structures and positive staining for Bcl-2 in spindle cells.
The patient demonstrated favorable postoperative progress and was discharged on postoperative Day 13 without any significant complications. Subsequent abdominopelvic CTs conducted 3 and 6 months after the surgery revealed no evidence of tumor recurrence and no changes in the right adrenal gland mass.
DISCUSSION
Pancreatic hamartoma is a benign tumor of the pancreas, and it accounts for an extremely small portion of pancreatic tumors. Most cases, including the present, have been pathologically confirmed after surgical resection to rule out malignancy [3]. This demonstrates the difficulty of differential diagnosis owing to the nonspecific findings in imaging studies such as CT and MRI. Indeed, there is a lack of consensus regarding the imaging findings for pancreatic hamartoma, and only case-series studies have been performed. Previous studies summarizing the cases to date have reported a diverse range of findings from imaging studies, including tumor size (ranging from 0.9 to 19 cm), nature (solid in 14 cases vs. solid/cystic in 14), and site of occurrence (head in 20 cases, body and tail in eight, and diffuse in two) [3, 4, 8]. Given the exceptionally low occurrence rate and the lack of specific diagnostic findings associated with pancreatic hamartoma, it is not surprising that most cases have been diagnosed based on postoperative pathologic examination.
The coexistence of tumor masses in both the pancreas and adrenal glands can be associated with multiple endocrine neoplasia type 1 (MEN-1) syndrome. While MEN-1 syndrome is known to affect the parathyroid, pituitary, and pancreas in typical cases, adrenal gland involvement has also been reported, with prevalence rates ranging from 9% to 45% [9]. In the present case, the patient had a 3.3-cm-sized mass in the pancreas and a 1.6-cm-sized mass in the right adrenal gland, confirmed by multiple imaging studies. In relation to MEN-1 syndrome, this co-occurrence may be explained by association of growth factor secretion by the pancreatic neuroendocrine tumor with a higher rate of adrenal tumor [10]. Herein, given that a neuroendocrine tumor is the typical presentation of MEN-1 in the pancreas, the decision to conduct PPPD with the initial impression of a pancreatic neuroendocrine tumor was reasonable.
In conclusion, we presented a case of pancreatic hamartoma with primary hyperaldosteronism in relation to an adrenal incidentaloma. The rarity of pancreatic hamartomas and their nonspecific findings on imaging studies complicate their differential diagnosis. For these reasons, we discussed the possibility of MEN-1 syndrome as an underlying cause, given the presence of tumor masses in both the pancreas and adrenal gland. Further research on this rare entity may improve future diagnosis and management for such patients.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
DATA AVAILABILITY
Participant data cannot be publicly opened in consideration of patient privacy concerns but can be shared by the corresponding author (Sung Hyun Kim; ohliebe@yuhs.ac) if a researcher provides a reasonable request.
References
Author notes
Jae Han Kim, Seung Hyun Yoon, Ji Ahn Choi and Ji Hyeon Kwak contributed equally to this report as co-first authors.
- adrenal glands
- aldosterone
- adrenal mass
- multiple endocrine neoplasia type 1
- pancreatic neoplasms
- pancreaticoduodenectomy
- surgical procedures, operative
- diagnostic imaging
- neoplasms
- pancreas
- benign pancreatic neoplasms
- pathology
- pylorus
- islet cell tumor
- adrenal incidentaloma
- excision
- pancreatic hamartomas



