-
PDF
- Split View
-
Views
-
Cite
Cite
Amy Yang, Siven Seevanayagam, Left brachiocephalic venous thrombus initially presenting as acute aortic syndrome, Journal of Surgical Case Reports, Volume 2023, Issue 8, August 2023, rjad461, https://doi.org/10.1093/jscr/rjad461
Close - Share Icon Share
Abstract
Upper extremity deep venous thrombosis (UEDVT) is rare but carries significant morbidity. Primary UEDVT presents non-specifically and there are no clear diagnostic or management guidelines, which are essential for early treatment to prevent potentially devastating complications such as pulmonary embolus or post-thrombotic pain syndrome. A patient with left brachiocephalic vein UEDVT initially diagnosed radiographically as an acute aortic syndrome and referred to a cardiothoracic unit is presented. Computed tomography venogram confirmed the diagnosis of UEDVT and therapeutic anticoagulation was started. This case highlights the need for validated diagnostic and management algorithms for UEDVT. Furthermore, this relatively rare condition should be considered for patients with acute chest pain and abnormal imaging referred to surgical units.
INTRODUCTION
Upper extremity deep venous thrombosis (UEDVT) is a rare and accounts for 4–10% of deep venous thrombosis cases [1]. UEDVT presents non-specifically with upper limb swelling, pain and neurological symptoms [2]. Primary UEDVT is due to repetitive effort-related thrombosis (Paget–Schroetter syndrome). Secondary UEDVT is more common and is associated with risk factors such as trauma, instrumentation, malignancy and pro-thrombotic conditions [3]. There are limited data regarding diagnosis and management of primary UEDVT [4]. In patients with minimal risk factors, diagnosis can be delayed due to lack of clinical suspicion. Untreated UEDVT is associated with considerable morbidity, including pulmonary embolism and a disabling post-thrombotic pain syndrome.
CASE REPORT
A 45-y-old woman presented to the Emergency Department with a one-week history of constant left-sided chest pain. She had no other cardiorespiratory symptoms, upper limb or facial swelling, or neurovascular symptoms. She had no past medical history, no regular medications and was a non-smoker who did not drink alcohol. There was no personal or family history of aortic pathology, autoimmune conditions, connective tissue disorders, malignancy or thrombophilia. On examination, she had a blood pressure of 180/80 mmHg. All other vital signs were normal. There was a mild neutrophilia (WCC 15.5 × 109/L), raised D-dimer (2353 ng/mL) and mildly elevated troponin I (41 ng/L). Other blood results were normal.
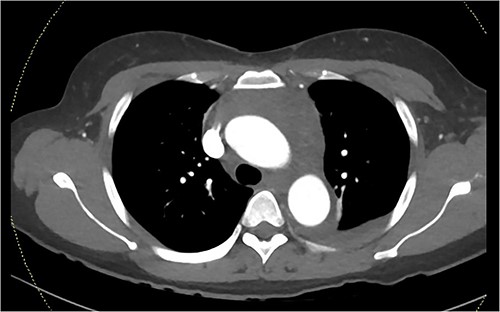
A computed tomography pulmonary angiogram (CTPA) was performed in the Emergency Department. Marked cresenteric aortic thickening from the level of the origin of the brachiocephalic trunk to the aortic hiatus in the diaphragm was noted, seen in Fig. 1. The CTPA was subsequently reported as an acute aortic intramural haematoma. The patient was transferred to the intensive care unit for blood pressure control with intravenous labetalol and cardiac monitoring.
On repeat review of the CTPA, imaging was deemed atypical for acute aortic syndrome. A CT aortogram with delayed phase was performed, which demonstrated a 66-mm upper anterior mediastinal soft tissue density compressing the left brachiocephalic vein, as seen in Fig. 2.
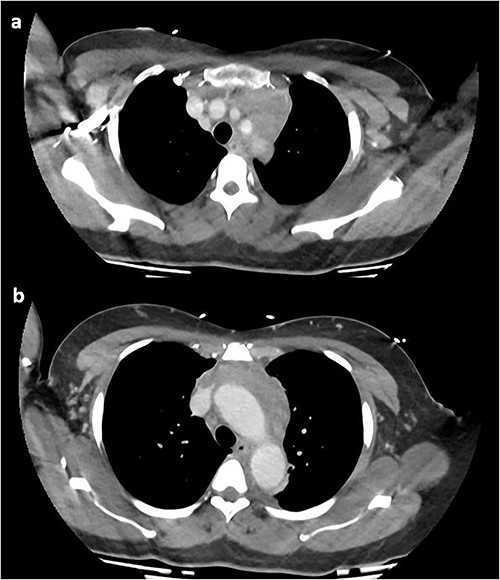
CT chest with contrast demonstrating anterior mediastinal soft tissue density in the (a) upper chest and (b) lower chest.
At this time, the mass was suspicious for a malignancy, possibly a thymoma, lymphoma or germ cell tumour. All serum tumour markers (AFP, CEA, CA19-9, CA125) were negative. FDG-PET demonstrated that the anterior mediastinal density had moderate FDG update and no other avid lesions elsewhere. After multidisciplinary discussion, the mediastinal density was thought to most likely represent left brachiocephalic venous thrombophlebitis. A dedicated computed tomography (CT) venogram was performed, demonstrating extensive UEDVT from the left internal jugular vein extending into the left brachiocephalic and subclavian veins, seen in Fig. 3.
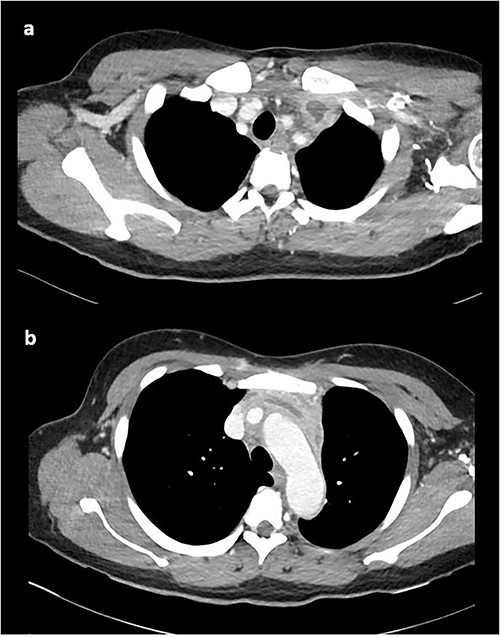
CT venogram demonstrating UEDVT in left brachiocephalic vein in the (a) upper chest and (b) lower chest.
On direct questioning, the patient recalled extensive upper arm activity preceding the chest pain. She was diagnosed with primary UEDVT and started on an oral anticoagulant (apixaban 10 mg twice daily for 1 week and 5 mg twice daily ongoing) and discharged home with oral antihypertensives. Thrombophilia screen was negative (protein C, protein S, antithrombin III, beta 2 glycoprotein antibody, cardiolipin antibody, lupus screen, prothrombin gene mutation, factor V Leiden).
Repeat CT venogram at 3 months demonstrated improvement in the thrombophlebitis and decrease in the size of the UEDVT. Chest pain and hypertension had completely resolved at the follow-up review after 1 month.
DISCUSSION
Primary UEDVT classically occurs in fit, young patients with a history of vigorous overhead arm exercise. It can be a manifestation of underlying venous thoracic outlet syndromem, where anatomical abnormalities of the first rib, clavicle or hypertrophy of the anterior scalene or subclavius muscles and repetitive microtrauma through aggravating movements (lifting, shoulder hyperabduction and external rotation) activates the coagulation cascade to cause thrombosis [5].
UEDVT can be present with chest or arm pain, upper limb and facial swelling, paraesthesia or fevers. Acute severe occlusive thrombus can also cause superior vena cava syndrome. These symptoms are non-specific, so further testing is required to confirm or exclude the diagnosis. There are no validated diagnostic guidelines for UEDVT. Constans et al. have developed a scoring system to assess the pre-test probability of UEDVT, demonstrated in Table 1 [6], which has a sensitivity of 78% and specificity of 64%. Elevated D-dimer has a specificity of 47% and sensitivity of 96% in diagnosing UEDVT but is not routinely recommended due to low specificity [7]. A combination Constans score, D-dimer and ultrasound is safe and efficacious in ruling out DVT, but this approach has not yet been validated [8].
Constans score for pre-test probability of upper extremity deep venous thrombus (UEDVT) [6].
| Item . | Points . | . |
|---|---|---|
| Venous material (catheter or access device in the subclavian or jugular vein, or pacemaker wire) | +1 | |
| Localized pain | +1 | |
| Unilateral pitting oedema of the extremity | +1 | |
| Other diagnosis at least as likely | −1 | |
| Classification | Points | Prevalence of UEDVT |
| Low probability | 0 | 9–13% |
| Intermediate probability | 1 | 20–38% |
| High probability | ≥2 | 64–70% |
| Item . | Points . | . |
|---|---|---|
| Venous material (catheter or access device in the subclavian or jugular vein, or pacemaker wire) | +1 | |
| Localized pain | +1 | |
| Unilateral pitting oedema of the extremity | +1 | |
| Other diagnosis at least as likely | −1 | |
| Classification | Points | Prevalence of UEDVT |
| Low probability | 0 | 9–13% |
| Intermediate probability | 1 | 20–38% |
| High probability | ≥2 | 64–70% |
Constans score for pre-test probability of upper extremity deep venous thrombus (UEDVT) [6].
| Item . | Points . | . |
|---|---|---|
| Venous material (catheter or access device in the subclavian or jugular vein, or pacemaker wire) | +1 | |
| Localized pain | +1 | |
| Unilateral pitting oedema of the extremity | +1 | |
| Other diagnosis at least as likely | −1 | |
| Classification | Points | Prevalence of UEDVT |
| Low probability | 0 | 9–13% |
| Intermediate probability | 1 | 20–38% |
| High probability | ≥2 | 64–70% |
| Item . | Points . | . |
|---|---|---|
| Venous material (catheter or access device in the subclavian or jugular vein, or pacemaker wire) | +1 | |
| Localized pain | +1 | |
| Unilateral pitting oedema of the extremity | +1 | |
| Other diagnosis at least as likely | −1 | |
| Classification | Points | Prevalence of UEDVT |
| Low probability | 0 | 9–13% |
| Intermediate probability | 1 | 20–38% |
| High probability | ≥2 | 64–70% |
Ultrasound (compression ultrasonography or colour Doppler) is the initial imaging modality of choice for UEDVT. Sensitivity and specificity of ultrasound alone remains unclear due to underpowered studies [9]. CT or magnetic resonance (MR) venography is often used as second-line if ultrasound is inconclusive. Digital subtraction venography is gold-standard for imaging, but it is invasive and carries procedural risks, so is reserved for those with suspected venous abnormalities or equivocal non-invasive imaging.
To date, no randomized controlled trials have evaluated the management of primary UEDVT [10]. Therapeutic anticoagulation, catheter-directed thrombolysis and surgical decompression are the mainstays of treatment. Oral anticoagulants, therapeutic low-molecular weight heparin and warfarin are all used and largely equivalent [11]. The duration of anticoagulation for primary UEDVT is usually 3 months. Catheter-directed thrombolysis is considered for those with acute and moderate to severe symptoms. UEDVT symptoms improve after thrombolysis [12], but there are relatively high bleeding rates and no significant improvement in post-thrombotic syndrome. Patients who underwent surgical decompression for UEDVT after thrombolysis (first rib resection, scalenectomy or division of anomalous bands), compared with thrombolysis alone, had better symptom relief and lower rates of recurrence [13]. The timing of surgery after thrombolysis remains controversial—early and late surgery are equal based on limited available evidence [14]. No surgical society has published official guidelines on surgical decompression in VTOS.
UEDVT is an important clinical entity that surgical units should be aware of as it may present similarly and appear radiologically like an acute aortic syndrome. Validated diagnostic and management guidelines should be developed for UEDVT, particularly regarding the role of surgical decompression in thoracic outlet obstruction.
AUTHORS' CONTRIBUTIONS
Dr Amy Yang performed the literature review and wrote the manuscript. A/Prof Seevanayagam contributed to the manuscript and supervised the review. Both authors read and approved the final version of the manuscript.
PATIENT CONSENT
Written informed consent was obtained from the patient.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest to disclose.
FUNDING
None.
DATA AVAILABILITY
Data is available upon request.



