-
PDF
- Split View
-
Views
-
Cite
Cite
Paola Solis-Pazmino, Mishel A Carvajal, Mikaela García, Richard Godoy, Camila Pazmino-Chavez, Cristhian Garcia, Managing thyroid cancer in Steinert's disease: the role of radiofrequency ablation, Journal of Surgical Case Reports, Volume 2023, Issue 6, June 2023, rjad381, https://doi.org/10.1093/jscr/rjad381
Close - Share Icon Share
Abstract
Radiofrequency ablation (RFA) is a minimally invasive, non-surgical technique used to treat benign or microcarcinoma thyroid nodules (TN) that provides an alternative for patients considered high-risk candidates for surgery. Myotonic dystrophy type 1 (DM1), also known as Steinert's Disease, is a multisystem disorder that affects various organs and tissues, including the thyroid. In this case, we presented a male patient diagnosed with DM1 who incidentally discovered a left TN with features indicative of thyroid cancer. Due to the patient's increased surgical risk associated with DM1, we opted for RFA as the treatment approach. In the follow-up, the TN decreased by 76.92% in size. The patient's thyroid function remained standard, with no reported complications or adverse effects post-treatment.
INTRODUCTION
Steinert's Disease, also known as Myotonic dystrophy type 1 (DM1), is an autosomal dominant multisystem disorder that affects various organs and tissues, including the thyroid [1]. It is characterized by muscle weakness and myotonia, and its prevalence is higher in Europe, ranging from 1 in 7400 to 1 in 10 700 individuals [2]. One of the notable manifestations of DM1 in the thyroid is the likelihood of finding elevated levels of thyrotropin (thyroid-stimulating hormone) and positive thyroid peroxidase antibodies. These abnormalities indicate an increased prevalence of thyroid nodules (TN) in DM1 patients, regardless of whether the nodules are benign or malignant [3].
Patients with DM1 often present with multiple comorbidities and are more susceptible to experiencing myotonias, respiratory depression, arrhythmias, neuromuscular blockade and post-anesthetic complications such as cardiorespiratory arrest [4]. Regarding managing TN in DM1 patients, radiofrequency ablation (RFA) has emerged as an effective non-surgical and minimally invasive technique (MIT). RFA is a suitable alternative for treating benign or microcarcinoma TN that pose a high risk for surgical interventions. RFA can precisely target and destroy the nodules using controlled thermal energy, resulting in fibrosis and a size reduction.
By considering the higher surgical risk associated with DM1 [5], RFA provides a safer and well-tolerated treatment option. It can alleviate symptoms, prevent further growth or malignant transformation of TN, and preserve thyroid function [6]. Regular follow-up and monitoring after RFA are crucial to assess treatment effectiveness and ensure the absence of late complications in these patients [7].
CASE REPORT
A young man, born in Quito, Ecuador, with a family history of DM1 (mother and maternal grandfather), was diagnosed with Steinert's disease in 2019 through molecular-genetic testing of dystrophia myotonic–protein kinase. A multidisciplinary team conducted thorough screenings to evaluate potential complications associated with DM1 comprehensively.
In May 2022, a thyroid ultrasound revealed a solid isoechoic left lobe TN measuring 12 × 11 × 13 mm, characterized by microcalcifications and irregular borders (Fig. 1). The color-flow Doppler ultrasound indicated increased perfusion, leading to its classification as a TI-RADS 5 nodule (Fig. 2). Elastography yielded a value of 2.2 (Fig. 3). Fine-needle aspiration confirmed Bethesda VI, consistent with thyroid papillary carcinoma.
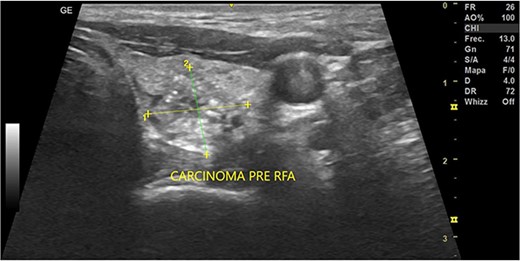
Thyroid ultrasound: transversal view, left lobe nodule identification and measures. Pre-RFA procedure.
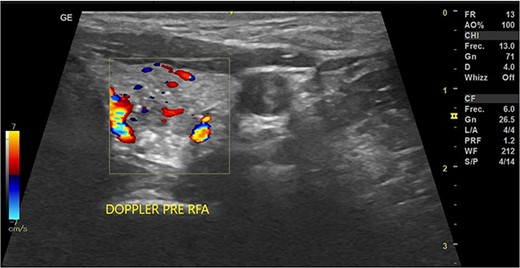
Doppler thyroid ultrasound: transversal view, left lobe nodule doppler. Pre-RFA procedure.
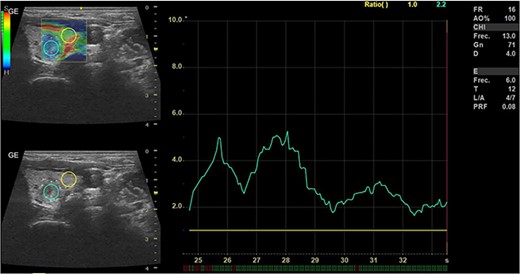
Thyroid ultrasound: elastography, Left lobe and nodule. Pre-RFA procedure.
Given the patient's diagnosis of Steinert's disease, he opted for RFA as a treatment approach for the TN. The RFA procedure was successfully performed by a head and neck surgeon using local anesthesia with 2% lidocaine without epinephrine. The trans-isthmic approach and moving shot technique were employed, utilizing RF Medical equipment with an 18G needle and 7 mm tip. No complications were encountered during the procedure, and the patient could return home within an hour.
During the first-month follow-up, the TN demonstrated a reduction in size to 10 × 14 × 15 mm with a volume of 1.11 cc, indicating a 22% increase in volume compared to the pre-RFA measurements (Fig. 4). Notably, the patient's thyroid function remained preserved.
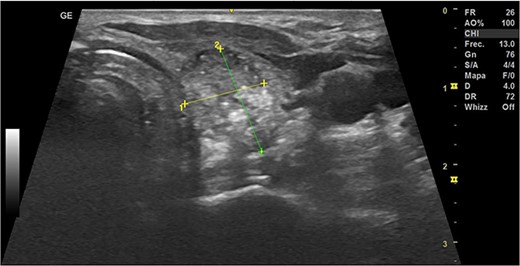
Thyroid ultrasound: left lobe, transverse view, nodule identification and measures. The first-month follow-up.
In January 2023, a subsequent ultrasound examination revealed a hypoechoic TN measuring 6 × 6 × 11 mm with a volume of 0.1 cc. This represented a significant decrease of 76.92% in size since the RFA procedure, and the color-flow Doppler ultrasound did not detect any abnormalities.
DISCUSSION
DM1 is a multisystem disorder characterized by muscle weakness, myotonia and a higher risk of comorbidities. Routine tests for DM1 patients may reveal incidental findings like TN.
The incidence of thyroid cancer, particularly papillary thyroid microcarcinoma (PTMC), is increasing globally. While thyroid surgery is the standard treatment for PTMC, MIT have emerged as alternative options. RFA is the most effective and safe MIT for solid TN and PTMC, offering a non-surgical alternative for high-risk patients. RFA is an outpatient image-guided thermal ablative procedure initiated in Asia and has expanded to Europe and South America. Moreover, it has emerged as an option for patients with high-risk surgery [8]. In addition, RFA is being studied as an alternative treatment for primary thyroid microcarcinoma. In 2017, The Korean Society of Thyroid Radiology included this intervention, in the RFA guidelines, as an alternative for patients that cannot undergo surgery or those who reject it [8]. A prospective study conducted by Zhang et al. [9] reported that 98 RFA-treated patients with MPTC showed 95.8% resolutions of nodules over 12 months with no recurrences or residual tumor tissue Hongyin et al. [10] evaluated the efficacy and safety of RFA in 95 patients over 55 with low-risk PTMC with a 1, 3, 6 and 12-month follow-up, showing the disappearance of the TN.
A comparative study evaluated the quality of life of patients with PTMC who underwent RFA compared to those who underwent surgery (total or partial thyroidectomy). This study showed that the patients who underwent RFA had no complications, a faster recovery, a better self-perception due to the absence of a scar, and fewer secondary symptoms than surgery [11]. Our patient experienced a faster and complication-free recovery after the procedure, and he feels comfortable with his appearance due to not having a scar.
Finally, a study carried out in Indonesia showed that RFA helps reduce health care costs since it is an outpatient procedure, hospital beds are not required, it can be performed outside the operating room with local anesthesia, the duration of the course is short, fewer personnel are required to carry out the procedure (operator and assistant), and patients have less anxiety about it [12].
CONCLUSIONS
RFA has emerged as an effective treatment option for benign NT and PTMC. It resolves thyroid lesions, alleviates compression symptoms and preserves thyroid function without causing life-threatening complications. RFA is particularly beneficial for patients who prefer non-surgical approaches or those with contraindications for surgery.
The field of RFA continues to expand, with ongoing studies exploring its application in various medical conditions. Further research and development are necessary to establish optimal protocols that ensure the adequacy, efficacy and cost-effectiveness of RFA as a treatment option for patients with different healthcare needs.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
COMPETING INTERESTS
The authors have no relevant financial or non-financial interests to disclose.
AUTHORS’ CONTRIBUTIONS
Paola Solis-Pazmino and Mishell Carvajal contributed equally to the study’s conception and design. Material preparation, data collection and analysis were performed by Paola Solis-Pazmino, Mishel Arboleda Carvajal, Mikaela García, Richard Godoy, Camila Pazmino-Chavez and Cristhian Garcia wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
ETHICAL APPROVAL
Informed consent was obtained from the patients.
CONSENT TO PARTICIPATE
Informed consent was obtained from the patient.
References
Author notes
P. Solis-Pazmino and M. A. Carvajal contributed equally.



