-
PDF
- Split View
-
Views
-
Cite
Cite
Caitlin E Kulig, Zamaan Hooda, Elissa Lebow, Luis Cerda, Bledi Zaku, Acute withdrawal in the postoperative setting in a patient taking standard therapeutic doses of pregabalin, Journal of Surgical Case Reports, Volume 2023, Issue 6, June 2023, rjad375, https://doi.org/10.1093/jscr/rjad375
Close - Share Icon Share
Abstract
Pregabalin is a gamma-aminobutyric acid analog that binds to voltage-gated calcium channels within the central nervous tissues, inhibiting the release of many excitatory neurotransmitters. It is used to treat various conditions including postherpetic neuralgia and diabetic peripheral neuropathy. Recently, its use has increased as part of non-opioid pain management algorithms. Prolonged use in high doses of pregabalin is associated with physical dependency and abuse, which can be seen when the medication is abruptly stopped. This phenomenon has been seen in studies focused on patients having abused or grown dependent on pregabalin. However, this has not been documented in patients taking therapeutic levels in the perioperative setting. This case report highlights a patient who experienced acute withdrawal symptoms of pregabalin after coronary artery bypass and aortic root enlargement.
INTRODUCTION
Pregabalin is a gamma-aminobutyric acid (GABA) analog that binds with high affinity to the alpha2–delta site of voltage-gated calcium channels within the central nervous tissues [1, 2]. By modulating calcium influx within the nerve terminals, pregabalin inhibits the release of multiple excitatory neurotransmitters, including glutamate, serotonin, substance P and calcitonin gene-related peptide, among others (see Fig. 1). Originally approved by the United States Food and Drug Administration in 2004, pregabalin is permitted to treat neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia, fibromyalgia, neuropathic pain associated with spinal cord injury and as an adjunct for the treatment of partial onset seizures [2–4]. However, pregabalin is also commonly used off-label for a variety of other indications, such as chronic refractory cough, generalized anxiety disorder, chronic pruritus and restless legs syndrome, among others [1, 5].
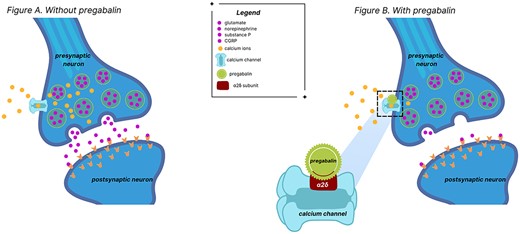
Over recent years, there has been an increase in the prescribing of pregabalin as part of multimodal or non-opioid pain management algorithms and has consistently been in the top 100 prescribed medications within the United States. Pregabalin is a federally controlled schedule V medication, as prolonged use at high doses may lead to physical dependence and abuse. Risk factors for pregabalin abuse include patients with a history of polysubstance abuse disorder [1]. Though uncommon at therapeutically prescribed doses, the increased prescribing and use of pregabalin has also led to a rise in widespread abuse; given the risk of physical addiction and dependency, withdrawal can occur when pregabalin is abruptly stopped, especially if patients are taking doses higher than therapeutically prescribed [2, 3]. Symptoms associated with pregabalin withdrawal are generally excitatory in nature, as the excitatory neurotransmitters such as glutamate rise rapidly; symptoms can include, but are not limited to increased seizure frequency in patients with seizure disorders, insomnia, headache, nausea, anxiety, diarrhea and hyperidrosis. Although there are data to support abrupt cessation of pregabalin causing withdrawal symptoms, these previous studies have primarily concentrated on patients having abused or grown dependent on pregabalin [6, 7]. In contrast, there have been very few reports regarding patients taking regular therapeutic doses of pregabalin that experience withdrawal symptoms, and there is no known literature to the authors’ knowledge regarding such cases in the perioperative setting. This case report describes a patient who experienced acute withdrawal symptoms of pregabalin after coronary artery bypass and aortic root enlargement.
CASE REPORT
A 61-year-old Caucasian female presented to the emergency department with a chief complaint of dyspnea on exertion. The patient had a weight of 48.5 kg and height of 157 cm. The patient had a past medical history of severe peripheral vascular disease (status post bilateral lower extremity stenting 5 years prior), type 1 diabetes, hypertension, smoking and hyperlipidemia. The patient underwent coronary angiogram which revealed 60% stenosis of the proximal left anterior descending (LAD), 70% mid LAD stenosis, 85% stenosis of the mid segment right coronary artery, as well as severe aortic valve stenosis, with a valve area of 0.7–0.8 cm2, Vmax 4.1 m/s and mean gradient of 37 mm Hg. The patient was referred to cardiothoracic surgery for evaluation of possible aortic valve replacement and coronary artery bypass grafting (CABG). The patient’s home medications included acetaminophen, aspirin, atorvastatin, biotin, vitamin D3, cilostazol, an insulin pump, lisinopril and pregabalin 200 mg by mouth three times daily, which was prescribed for her diabetic neuropathy.
At baseline, the patient was alert and oriented and able to follow commands. After appropriate preoperative risk stratification, the patient underwent CABG as well as aortic root enlargement and aortic valve replacement.
The patient was recovered in the intensive care unit and was extubated on postoperative day 2 (POD 2). The patient became agitated, anxious and difficult to redirect, though was able to follow commands. A bedside echocardiogram was performed, which revealed no aortic regurgitation and the bioprosthetic aortic valve to be in proper position. Investigating pain as a potential cause of the patient’s disorientation and agitation, IV opioids were trialed with minimal improvement. Given her history of diabetes, hypoglycemia or other disturbances in her blood glucose level was also immediately ruled out. Throughout the course of the day, the patient’s agitation and restlessness worsened and the patient became increasingly more difficult to redirect. She was also noted to have intermittent episodes of drowsiness and her blood pressures continued to fluctuate between episodes of agitation, delirium and somnolence. In order to resolve the patient’s distress, the patient was trialed on haloperidol, lorazepam and quetiapine, which only temporarily and mildly resolved her distress. Ultimately, light sedation with dexmedetomidine was required to prevent harm to both the patient and staff and the patient was ordered for a 1:1 sitter. Upon further investigation, it was found that the patient had a history of smoking, and a nicotine patch was ordered and started the morning of POD 2. However, 24 h after patch placement, on POD 3, the patient continued to experience continually disorientation, forgetfulness and agitation despite maximal doses of dexmedetomidine.
At this time, further investigation was done to identify other causes of the patient’s agitation and delirium, though were all unremarkable. Glucose continued to remain within normal limits, blood urea nitrogen was only slightly elevated at 40 mg/dL, liver function, arterial blood gas, and electrolytes were normal and there were no clinical signs or symptoms of an acute infection or intracranial process. It was also confirmed that the patient was experiencing regular circadian cycles with light (e.g. blinds were open during the day, closed at night). Upon consultation from the clinical pharmacist, the patient’s home medication list and medical history was reviewed, and pregabalin was identified to be a potential cause, as the patient had not started her home pregabalin in the hospital.
Benzodiazepines were then stopped as they can exacerbate delirium, especially in older patients. Pregabalin 200 mg by mouth three times a day was restarted. Her scheduled quetiapine 50 mg by mouth daily, olanzapine 5 mg intramuscularly as needed and dexmedetomidine drip were continued in the meantime, though the patient did not end up requiring any more doses of olanzapine. The first dose of pregabalin 200 mg was given on POD 3 at 20:38, and by 8:44 on POD 4, the patient was alert and oriented x4 without any complaints, with all sedation with dexmedetomidine stopped. The patient’s blood pressure also began to stabilize. As her postoperative course continued, the patient expressed desire to begin cardiovascular rehabilitation. Two days after restarting the pregabalin, the patient was discharged to home, without any further prescriptions for adjunctive medications such as quetiapine. The patient was seen in the cardiothoracic surgery office shortly after her discharge. Although she mentioned generalized fatigue, she expressed improvement in her appetite and determination to continue cardiovascular rehabilitation. She was deemed to be recuperating well from her surgery and the patient was advised to steadily increase daily activities as tolerated.
DISCUSSION
In this report, we have highlighted a case of acute pregabalin withdrawal in the postoperative setting in a patient without a history of substance abuse taking therapeutic doses of pregabalin.
As previously mentioned, there is minimal literature to describe this clinical phenomenon, and this case report demonstrates relatively severe pregabalin withdrawal can occur within days of discontinuation in the perioperative setting. The symptoms that the patient in our report experienced included psychological symptoms of disorientation, restlessness, anxiety and agitation, even at maximal doses of sedation with dexmedetomidine. At the time, delirium was suspected and laboratory workup revealed no abnormalities. When reviewing the patient’s medication list, it was noted that her pregabalin had not been reinitiated and her symptoms resolved shortly after its administration. Considering that our patient had no underlying psychiatric disorder, other underlying causes of delirium were ruled out, and her symptoms subsided within hours of restarting pregabalin, it is reasonable to suspect that she experienced pregabalin withdrawal.
It is worth noting that the patient in this case report experienced pregabalin withdrawal even though she was on a standardly prescribed, therapeutic dose of pregabalin at home. Though there is more evidence supporting acute pregabalin withdrawal at doses exceeding therapeutic threshold, there is a dearth (paucity)of literature in which patients experienced withdrawal at standard therapeutic doses, especially in the postoperative setting.
This case report adds to the limited body of evidence that pregabalin withdrawal can be seen even in the setting of discontinuation of lower doses of the medication. Of note, when comparing this case to those already published, our patient in this report experienced withdrawal only days after discontinuation; in the previous cases, the patients were noted to develop withdrawal symptoms weeks to months after termination.
Based on our findings and information from prior reports, physicians and surgeons must be aware of the potential for pregabalin withdrawal after its cessation, even if the patient has no history of substance abuse and is taking low-to-regular doses. Though the exact mechanism of pregabalin withdrawal remains unclear, it may be because of an increase in excitatory neurotransmitters compared with the body’s baseline on the drug [8–11]. With this in mind, clinicians may consider a gradual taper of pregabalin rather than abrupt discontinuation in patients taking these low amounts of the medication, or continuing throughout the hospital stay. As there is limited literature on this topic, further research is clearly warranted to investigate the risk factors and mechanism that is associated with pregabalin withdrawal in those taking low-to-regular doses of pregabalin without history of polysubstance abuse. Data extrapolated from these studies can be directly applied to patients fitting this profile in the perioperative setting.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



