-
PDF
- Split View
-
Views
-
Cite
Cite
Kantaro Hara, Hikaru Miyamoto, Nao Furukawa, Takuya Kimura, Satsuki Soeda, Kazunori Okabe, Successful use of left ventricular support device (Impella 5.0) and extracorporeal membrane oxygenation (ECMO) for postoperative cardiac arrest after lung cancer surgery: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 5, May 2023, rjad258, https://doi.org/10.1093/jscr/rjad258
Close - Share Icon Share
Abstract
Postoperative hemodynamic support with an Impella 5.0 was effective in a man who underwent lung lobectomy for lung cancer and cardiogenic shock. A 75-year-old man presented to hospital with an abnormal chest shadow on radiography. After thorough examination, the patient was diagnosed with lung cancer, and left lower lobectomy was performed. On the 2nd postoperative day, the patient experienced cardiac arrest because of a sudden drop in saturation of percutaneous oxygen. After a third defibrillation, his heartbeat resumed, and he was intubated and placed on a ventilator. Coronary angiography revealed acute coronary syndrome and the patient fell into a state of shock, which required venoarterial extracorporeal membrane oxygenation (VA-ECMO) support. Nevertheless, the circulatory dynamics are unstable, and Impella 5.0 was introduced. VA-ECMO and the Impella 5.0 were discontinued on the 6th and 8th postoperative days, respectively. The patient was eventually transferred to a nearby facility for further rehabilitation 109 days later.
INTRODUCTION
Acute ischemic heart disease after lung cancer surgery is a rare but sometimes fatal complication. The fatality rate is particularly high when cardiac arrest occurs. In addition, patients who have undergone pneumonectomy are prone to circulatory instability because the pulmonary vascular bed is reduced compared with the preoperative level [1].
Herein, we report a case of a patient with acute coronary syndrome who had a cardiac arrest on the second day after robot-assisted left lower lobectomy for lung cancer, which was successfully treated with venoarterial extracorporeal membrane oxygenation (VA-ECMO) and Impella 5.0 insertion.
CASE REPORT
A 75-year-old male was referred to our hospital for preoperative examination of an abnormal chest shadow during orthopaedic surgery. Chest computed tomography (CT) revealed a mass nodule measuring 4.1 cm in S6 of the left lung, and the tracheobronchial lymph node (No 4 L) was enlarged (Fig. 1). Fluorodeoxyglucose positron emission tomography (PET) revealed abnormal accumulation with a maximum standardized uptake value of 20.4 at the mass, 9.4 at the lymph node and no metastases to other organs (Fig. 2). Based on the above findings, we diagnosed the patient with left lower lobe lung cancer (cT1bN2M0, c-Stage IIIA). The patient had no history of cardiovascular disease and was unaware of chest pain, such as an angina attack. We performed robot-assisted left lower lobectomy.
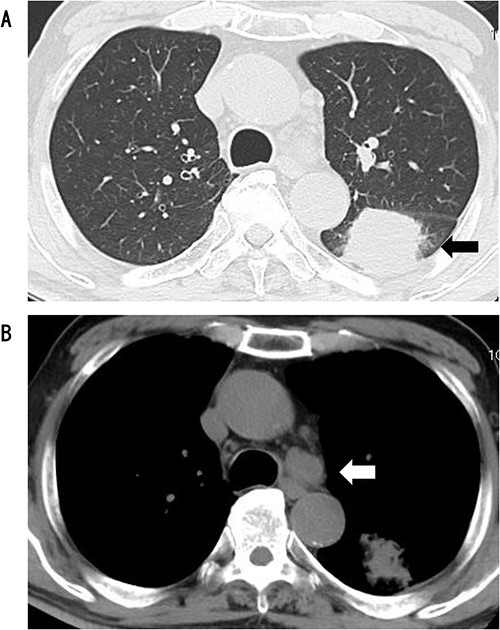
CT showed a circular mass measuring 4.1 cm in the S6 of the left lower lobe (A) and enlarged lymph node #4 (B).
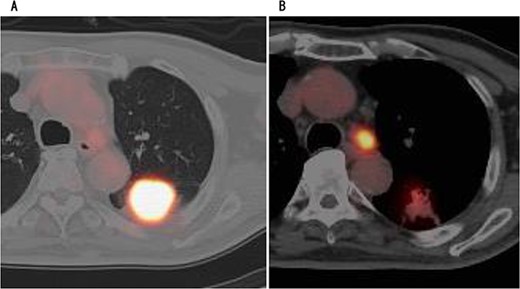
PET showed high accumulation in the mass (A) and lymph node (B).
On the 2nd postoperative day, the patient experienced cardiac arrest because of a sudden drop in the saturation of percutaneous oxygen, after which his heartbeat resumed and he was intubated and placed on a ventilator. Transthoracic echocardiography showed left ventricular ejection fraction of 30%, diffuse hypokinesis of left ventricular wall motion and asynergy. Electrocardiography showed complete right bundle branch block, but no ST-segment elevation. Blood tests showed marked elevation of myocardial desensitization enzymes (CK: 362 U/L, cardiac troponin: 1143 pg/ml). Since ischemic heart disease could not be ruled out, angiography was performed. Coronary angiography revealed acute coronary syndrome with 90% occlusion of the left circumflex branch and 100% occlusion of the left anterior descending branch (Figs 3 and 4). The patient experienced shock and required VA-ECMO. However, the circulatory dynamics were unstable, and intra-aortic balloon pumping (IABP) was introduced. Furthermore, because the blood pressure could not be maintained with circulatory support from the IABP, we converted it to Impella 5.0. Assisted circulation with Impella 5.0 stabilized the patient’s blood pressure and allowed us to open the left circumflex and left anterior descending branches. VA-ECMO initially required an auxiliary flow of 1.2 L/min, which could be gradually reduced; Impella 5.0 initially required an auxiliary level of P7, which was reduced to P5 after VA-ECMO removal. VA-ECMO and Impella 5.0 were discontinued on the 6th and 8th postoperative days, respectively. He was eventually transferred by walking to a nearby facility for further rehabilitation 109 days later. One year later, the patient was recurrence-free of lung cancer.
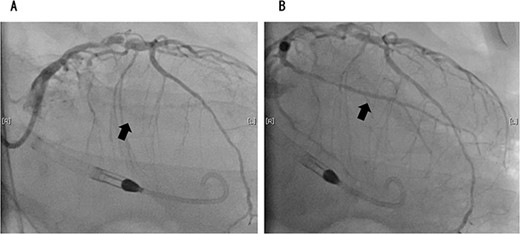
Coronary angiography showed 100% occlusion of the left circumflex branch (A). After the procedure, the occlusion was completely released (B).
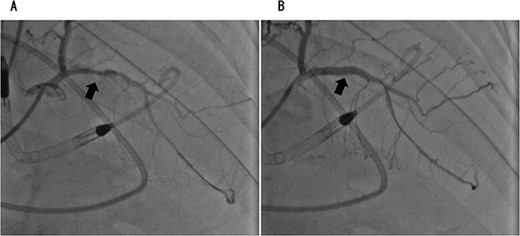
Coronary angiography showed 90% occlusion of the left anterior descending branch (A). After the procedure, the occlusion was completely released (B).
DISCUSSION
The Impella is a catheter-based blood pump that is inserted into the left ventricle through the femoral artery and motorized to remove blood directly from the left ventricle and deliver it to the ascending aorta to assist body circulation. Mechanical circulatory assistance using Impella can improve haemodynamic compromise in patients with acute heart failure by reducing the left ventricular load and maintaining peripheral organ perfusion. Compared with intra-aortic balloon pumps, Impella therapy increased heart rate and decreased left ventricular end-diastolic pressure to a great extent but had no positive effect on 30-day mortality in patients with acute myocardial infarction [2]. Nishikawa et al. [3] reported that short-term improvement in myocardial haemodynamics with Impella is useful in patients with Takotsubo syndrome and cardiogenic shock. Several recent reports have demonstrated the usefulness of Impella as a preoperative circulatory aid in cardiac surgery or as an adjunct to percutaneous procedures. In these reports, the adjunctive use of Impella prevented haemodynamic compromise and served as a bridge to the scheduled surgical repair of a ruptured ventricular septum after myocardial infarction [4, 5]. During Impella insertion, the haemodynamics are good; however, device-related complications, such as aortic valve injury, mechanical haemolysis, limb ischaemia, puncture-site injury and bleeding because of anticoagulation with heparin, should also be considered.
In contrast, the incidence of postoperative ischemic heart disease in patients with postoperative lung cancer has been reported to be below 0.1%, out of which 20% patients die [6]. In other words, it is a very difficult postoperative complication to save lives. Therefore, early diagnosis and treatment are essential. We report a rare case of Impella in a patient with severe heart failure after lung cancer surgery. In this case, despite the disadvantage of an increased bleeding risk because of the anticoagulation effect of Impella, the maintenance of circulatory function was essential for the maintenance of vital activity and was a desirable option.
CONCLUSION
We report a case of acute coronary syndrome resulting in cardiac arrest on the 2nd day after robot-assisted left lower lobectomy for lung cancer, which was saved by therapeutic intervention with VA-ECMO and Impella 5.0.
CONFLICT OF INTEREST STATEMENT
None declared.



