-
PDF
- Split View
-
Views
-
Cite
Cite
Hirotaka Kumeda, Gaku Saito, Utility of indocyanine green in assessing blood perfusion in anomalous systemic arterial supply to the lung, Journal of Surgical Case Reports, Volume 2023, Issue 4, April 2023, rjad209, https://doi.org/10.1093/jscr/rjad209
Close - Share Icon Share
Abstract
The chosen treatment for anomalous systemic arterial supply to the basal segment of the lung (ABLL) is typically the division of the anomalous artery and resection of the abnormal area, dependent on the anomalous artery. Although only division or interventional embolization of the anomalous artery may be selected. However, leaving the area dependent on the anomalous artery can lead to complications, such as necrosis and pulmonary infarction. We report the case of a 39-year-old female with ABLL. Intraoperatively, the anomalous artery was first divided. Indocyanine green (ICG) was subsequently injected intravenously to evaluate blood perfusion within the abnormal lung area. Since the abnormal area was still poorly perfused after a few minutes, a left basal segmentectomy was performed because of the possibility of complications. Thus, evaluation of perfusion via ICG can be used in the decision to resect abnormal area.
INTRODUCTION
An anomalous systemic arterial supply to the basal segment of the lung (ABLL) is a relatively rare congenital vascular anomaly. The basal segment of the lung receives blood from the thoracic descending aorta, and the bronchus is positioned at the normal branch. Because of the risk of complications such as haemoptysis, pulmonary hypertension and heart failure, treatment is required [1]. In addition to division of the anomalous artery and resection of the area dependent on the anomalous artery, other treatment options include division or interventional embolization of the anomalous artery [1–3]. However, there is a risk of complications, such as necrosis and pulmonary infarction. Therefore, the optimal treatment is a matter of debate.
We report a case of anomalous systemic arterial supply to the basal segment of the left lung. Left basal segmentectomy was performed after indocyanine green (ICG) confirmed poor blood perfusion of the abnormal area after the division of the anomalous artery.
CASE REPORT
A 39-year-old female, who typically experienced a small amount of haemoptysis about once a year was referred to our hospital because of a new case of severe haemoptysis. Chest computed tomography (CT) showed a pulmonary infiltrative shadow because of haemorrhage in part of the left basal lung segments (Fig. 1a). An anomalous artery branching from the descending aorta into the left basal lung and the position of the bronchus at the normal branch were observed, suggesting an ABLL (Fig. 1b). Three-dimensional CT showed the distribution of blood flow within the left lower lobe was divided between the original atrophic basal part of the pulmonary artery and the anomalous artery (Fig. 1c). While haemoptysis improved with conservative treatment, she requested surgery, which was performed 7 months following severe haemoptysis onset. Written informed consent was obtained from the patient.
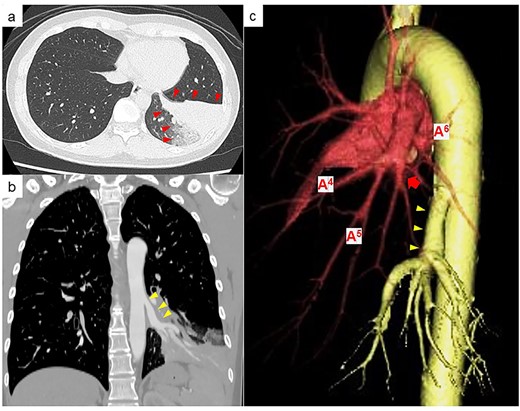
(a) Chest CT at the time of haemoptysis showing a pulmonary infiltrative shadow because of haemorrhage in part of the left basal lung segment (red arrowheads). (b) Contrast-enhanced CT showing an anomalous artery branching from the descending aorta (yellow arrowheads). (c) Three-dimensional CT showing that the original basal part of the pulmonary artery is atrophic (red arrow), and the basal part of the pulmonary artery and anomalous artery (yellow arrowhead) is clearly separated in the distribution of blood flow within the left lower lobe.
We performed complete video-assisted thoracic surgery. The vessels on the surface of the abnormal left basal segment were distended, and the boundary with the normal lung was clear (Fig. 2a). First, the anomalous artery branching from the aorta into the left basal segment was divided using a stapler (Fig. 2b and c). To assess blood flow in the left lung lobe, 0.25 mg/kg ICG was injected intravenously by near-infrared fluorescence imaging. Immediately after administration, the normal left lung area showed fluorescence. However, the abnormal basal lung area exhibited no fluorescence, this was still the case minutes later (Fig. 2d). Since the abnormal area had poor blood perfusion and could potentially become necrotic, we performed left basal segmentectomy. The patient had the chest tube removed 2 days after surgery and was discharged from our hospital 6 days after surgery. She was treated in-hospital setting until the wound pain improved to some degree, which have contributed to our relatively longer hospital stay. After discharge from the hospital, she had no major complications and is doing well in the following year.
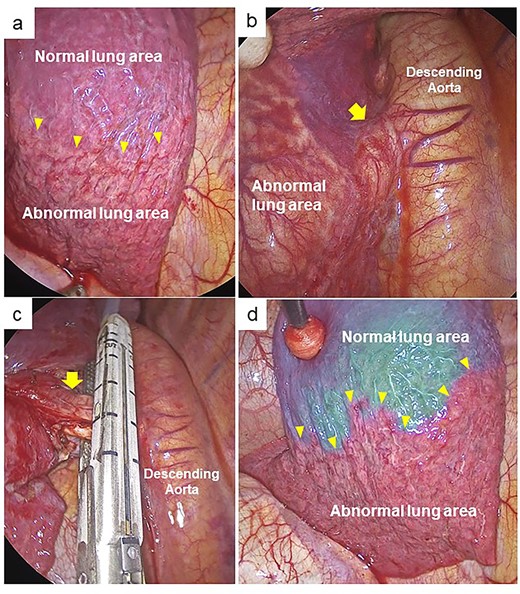
Intraoperative findings. (a) The thoracoscopic view showing clear boundary between the abnormal and normal lung area in the left basal lung (yellow arrowheads). (b) The anomalous artery (yellow arrow) was identified. (c) The anomalous artery (yellow arrow) was divided using a stapler. (d) Fluorescence navigation immediately after intravenous injection of ICG after dividing the abnormal artery. The abnormal basal lung area exhibited no fluorescence and was clearly separated from the normal lung area (yellow arrowheads).
DISCUSSION
ABLL is considered a different disease from pulmonary sequestration, as there is no anomaly in the bronchial trees and the venous drainage is normal. The left lower lobe is affected the most [1]. Some patients are asymptomatic; however, the most common symptom is haemoptysis. Additionally, pulmonary capillaries and veins under excessive pressure from left-to-left circulatory shunts from anomalous arteries are prone to rupture, causing intra-alveolar haemorrhage [4]. Because of the potentially fatal consequences of severe haemoptysis, pulmonary hypertension and heart failure, all patients, including asymptomatic ones, require surgical treatment [5, 6].
The chosen treatment for this disease is typically division of the anomalous artery and resection of the area dependent on the anomalous artery, such as lobectomy or basal segmentectomy [1, 4]. These treatments have the advantage of resecting abnormal lungs, including fragile vessels that cause haemoptysis. However, it can result in decreasing lung function and is highly invasive. Other treatments include division of the anomalous artery or interventional embolization, such as coil embolization [3]. These therapies are minimally invasive but have the disadvantage of potential necrosis, partial collapse of the remaining abnormal lung or severe pulmonary infarction [7, 8]. Particularly in the case of coil embolization, there have been reports of reopening and haemoptysis, and the long-term results are also unknown [3, 9].
Recently, ICG has been used in the field of pulmonary surgery, for example, in identifying demarcation lines during lung segmentectomy [10]. It is useful in the identification of the demarcation line in pulmonary sequestration or in ABLL [11, 12]. However, to date, no studies have used ICG to evaluate blood perfusion to determine the necessity of resection.
In our case, we evaluated blood perfusion in the abnormal lung, intraoperatively, by intravenous ICG after disconnecting the anomalous artery branching from the aorta into the left basal segment. While the normal left lung area exhibited fluorescence, the abnormal areas did not and most exhibited no fluorescence even after a few minutes. Therefore, a left basal segmentectomy was performed to reduce the possibility of postoperative necrosis or severe pulmonary infarction. If greater fluorescence area in the abnormal area was observed after a few minutes of ICG, resection of the basal lung might not have been performed. However, at present, there are no clear criteria for the extent of blood perfusion in an abnormal lung that does not require lung resection. Further accumulation and analysis of more cases are necessary in the future.
In conclusion, in ABLL, evaluation of blood perfusion by ICG after resection of the anomalous artery may part of the criteria in deciding whether a resection of the abnormal lung area is necessary.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.com) for English language editing.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



