-
PDF
- Split View
-
Views
-
Cite
Cite
Carlos Theodore Huerta, Kirby Quinn, Ricardo Restrepo, Madeleen Mas, Bhavi Patel, Steven J Melnick, Juan E Sola, Omaida C Velazquez, Chad M Thorson, Peripheral vascular bypass with cadaveric arterial allograft in a toddler with femoral mycotic aneurysm, Journal of Surgical Case Reports, Volume 2023, Issue 4, April 2023, rjad198, https://doi.org/10.1093/jscr/rjad198
Close - Share Icon Share
Abstract
Mycotic aneurysms are exceedingly rare in the pediatric population. The optimal surgical treatment for children with this disease is unclear as aneurysm resection and vascular reconstruction are uncommonly performed in young children. We present a unique case of a 21-month-old child with a complex cardiac history who presented with limb ischemia and was discovered to have thrombosis of the common femoral and superficial femoral artery. Groin exploration revealed a left common femoral and superficial femoral artery mycotic aneurysm that was successfully repaired with excision of the mycotic aneurysm, external iliac to profunda femoral artery vascular bypass using cryopreserved arterial allograft and femoral vein reconstruction. This case demonstrates successful vascular reconstruction can be performed in a young child with an Aspergillus mycotic aneurysm using cadaveric arterial allograft.
INTRODUCTION
Aspergillus infection is known to cause mycotic aneurysm formation. However, this phenomenon is exceedingly rare in the pediatric population. Furthermore, the need to perform lower extremity (LE) revascularization is rare in pediatric patients and is more likely to be due to trauma rather than aneurysmal formation [1, 2]. Thus, the optimal surgical approach is not well-documented. The gold standard of adult LE revascularization cannot be directly translated to children due to the immature nature of pediatric veins, sometimes yielding unsuitable conduits and the greater potential for future limb-length discrepancy [3].
In the absence of viable autologous conduits, the optimal revascularization technique in children is unknown. We report the case of a medically complex 21-month-old with mycotic aneurysms secondary to Aspergillosis and detail the successful repair of a large femoral aneurysm.
CASE REPORT
A male patient born via Cesarean section presented at day of life one due to Tetralogy of Fallot with multiple ventricular septal defects. After repair at 2 months of age, his postoperative course was complicated by a right middle cerebral artery mycotic aneurysm, which was resected. He was discharged home at 19 months old.
The patient was readmitted the following month at the age of 20 months due to recurrent fevers. A serum PCR (Karius©) test was positive for Aspergillus, and Voriconazole was initiated. Computerized tomography (CT) revealed an ascending aortic pseudoaneurysm for which he underwent surgical resection.
The patient became progressively non-weight bearing on the left LE and developed significant pain with hip adduction. An ultrasound Doppler demonstrated complete occlusion of the left common femoral artery with a thrombosed aneurysm and reconstitution of flow to the popliteal artery. CT angiographydemonstrated complete occlusion of the left common femoral artery (Fig. 1). He had weak dorsalis pedis pulses and biphasic signals in the ipsilateral dorsalis pedis and posterior tibial arteries. Due to persistent thrombosis on anticoagulation, loss of palpable pulses and change in Doppler signals, he was taken to the operating room. Preoperative vein mapping revealed an inadequate greater saphenous vein diameter (1 mm), and an absent right internal jugular vein (ligated after previous ECMO cannulation). The procedure began with a left retroperitoneal incision to obtain proximal control of the ipsilateral common, internal and external iliac vessels and a left groin incision to expose the femoral aneurysm. There was an extremely dense inflammatory response that obliterated a significant segment of common femoral vein with purulent material extruding from the region (Fig. 2A). The 2 mm profunda artery was the remaining outflow. A 6 mm cryopreserved cadaveric artery allograft (CryoArtery©) was utilized to perform the bypass between the external iliac artery and the profunda femoris artery in an end-to-side fashion using interrupted 5–0 Prolene® suture. The wound was closed in three layers using absorbable suture. The mycotic aneurysm was resected from the external iliac artery to the proximal superficial femoral artery. As the femoral vein was densely adherent to the inflammatory mass, a segment was removed and reconstructed with CryoArtery© (Fig. 2B). Inflammatory markers (preoperative C reactive protein 24 mg/l and procalcitonin 21 ng/ml) downtrended postoperatively (1.5 mg/l and 0.13, respectively at discharge). The patient was discharged 48 days postoperatively. Serial fungal tests were followed, and antifungal coverage was discontinued 1 year after discharge. As of 36 months follow-up, the patient is ambulating well with equal pulses and no evidence of limb-length discrepancy.
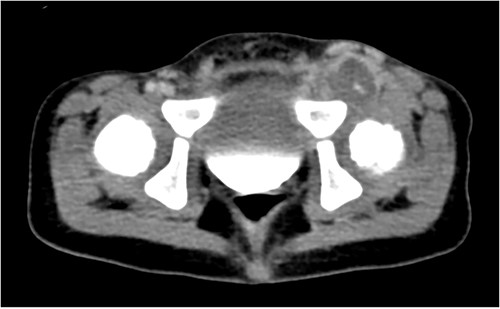
Axial computed tomography showing a large left common femoral artery mycotic aneurysm with a dense inflammatory reaction.
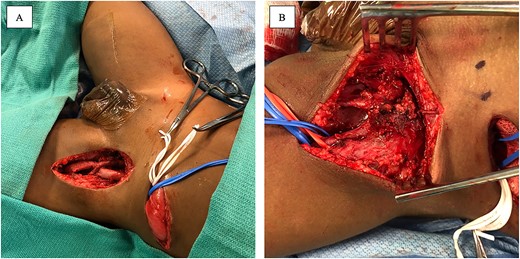
Intraoperative photograph demonstrating the completed anastomosis between the left common iliac artery and the profunda femoris artery, along with femoral vein reconstruction (A). Intraoperative photograph demonstrating large mycotic aneurysm involving the femoral artery (B).
DISCUSSION
Due to their rarity, treatment for peripheral arterial aneurysms are not well-documented in children [3, 4]. The mainstay of treatment in adults is surgical repair including aneurysm resection and vascular reconstruction [5, 6]. Determining the conduit for reconstruction is challenging in children due to small arterial diameter and immature nature of potential venous conduits [5]. Although an autologous conduit would have been a preferred bypass, it was not possible owing to the patient’s immature venous system and prior surgical manipulation. Prior reports show successful results of using a cryopreserved arterial allograft for infected prosthetic grafts as they are more resistant to reinfection [7, 8], which was favorable for our patient. However, these grafts may be more prone to a high reintervention rate within 5 years of the use for vascular prosthetic infection [9, 10].
Restoring flow to the distal extremity is crucial, not only to avoid future limb-length discrepancies, but also to maintain flow in the bypass conduit postoperatively [6]. Postoperatively, our patient received intravenous heparin immediately and was bridged to prophylactic enoxaparin for 3 months and aspirin indefinitely. Many adults are treated with a variable course of dual antiplatelet therapy with aspirin and clopidogrel after peripheral revascularization, and many trials recommend treatment with aspirin for 6 months or indefinitely along with clopidogrel for 1–3 months [11]. The optimal duration of antiplatelet therapy and dosage in children undergoing LE revascularization is unknown. One large series examining vascular reconstruction for traumatic injuries in 99 pediatric patients utilized a similar regimen of indefinite aspirin (81 or 325 mg based on weight [12]) in those with venous or synthetic conduits [13]. Of patients undergoing LE reconstruction (n = 24), only one experienced postoperative graft thrombosis during follow-up. Although surgical repair was successful in our patient, future research questions include not only the use of conduits (cadaveric artery, cadaveric vein, synthetic graft) [14], but also the duration and risks of long-term anticoagulation/antiplatelet therapy required in children [11].
Yet another component exemplifying this case’s rarity is the formation of mycotic aneurysms in a patient as young as 21 months. Mycotic aneurysms due to Aspergillus are exceedingly rare, especially in young children. Persistent Aspergillosis tends to disseminate into larger caliber blood vessels in the brain and heart [11], as demonstrated by our patient who presented with mycotic aneurysms in the right cerebral artery, ascending aorta and peripheral vasculature.
CONCLUSION
Repair of peripheral arterial aneurysms in children is not well-documented and inherently difficult in very young patients who lack an available mature venous conduits. If the use of an autologous conduit is not possible, we show here that a cadaveric arterial allograft can be a suitable and durable alternative.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None declared.
AUTHORS’ CONTRIBUTIONS
Study Conception and Design: C.T.H., K.Q., C.M.T. Acquisition of Data: All authors. Analysis and Interpretation of Data: All authors. Drafting of Manuscript: C.T.H., K.Q., C.M.T. Critical Revision of Manuscript: All authors.
PATIENT CONSENT
Informed consent to publish the patient’s case and images was obtained by the patient’s legal guardian. This report was deemed exempt from review by our Institutional Review Board. This report does not contain any personal information that could lead to the identification of the patient.
AUTHORSHIP
All authors attest that they meet the current ICMJE criteria for Authorship.
References
- thrombosis
- mycotic aneurysm
- aneurysm
- limb ischemia
- aspergillus
- child
- cryopreservation
- femoral artery
- femoral vein
- ilium
- pediatrics
- reconstructive surgical procedures
- surgical procedures, operative
- heart
- toddler
- excision
- allografting
- femoral artery, superficial
- vascular bypass
- incision and exploration of inguinal region



