-
PDF
- Split View
-
Views
-
Cite
Cite
Fawatim A Al Nahwi, Ali AlMomen, Sarah A Alkishi, Fadhel Almolani, Mohammed A Al Ameer, Aziza A Alzubaidi, Wejdan K Suwayyid, Abdullah Z Alkhars, The different clinical presentations of pediatric allergic fungal sinusitis, Journal of Surgical Case Reports, Volume 2023, Issue 4, April 2023, rjad149, https://doi.org/10.1093/jscr/rjad149
Close - Share Icon Share
Abstract
In this paper, we aim to study the different clinical presentations of pediatric allergic fungal sinusitis (AFS) in the Eastern Province of Saudi Arabia, and to review the experience in the diagnosis & management of AFS in children at King Fahad Specialist Hospital. This study is a retrospective case series of pediatric patients diagnosed and managed as AFS at a tertiary referral hospital in Saudi Arabia. The clinical presentation of pediatric AFS varies widely and includes unilateral, unilateral with proptosis, bilateral, alternating, isolated sphenoid and extensive with intracranial & intraorbital involvements. Children with AFS present with different clinical features when compared to adults. Therefore, they require a high index of suspicion for evaluation and early aggressive treatment.
INTRODUCTION
The primary classification of fungal sinusitis dividing it into invasive and non-invasive was developed in the last decade from a need to separate the two categories and reach a consensus on terminology [1, 2]. In like manner, the invasive fungal sinusitis was further subdivided into acute fulminant invasive, chronic invasive and chronic granulomatous fungal sinusitis. Meanwhile, non-invasive fungal sinusitis was subdivided into allergic fungal sinusitis (AFS) and fungal ball. This categorization of fungal sinusitis has been built based on distinctive histopathological features supported by characteristic clinical, radiological and mycological criteria [3–4]. AFS is considered a benign, non-invasive, extra-mucosal fungal sinus disease caused by a hypersensitivity reaction secondary to extra-mucosal fungus in the sinus [5]. The clinical course and presentation of AFS in children differ from adults and often tend to be more aggressive. Given that, it is fundamental for physicians to diagnose AFS as early as possible, to start management promptly without an unnecessary delay, followed by periodic endoscopic follow-ups post-operatively [6]. That is especially important when taking into consideration the fact that children, with their growing incompletely developed facial skeletons, are more likely to develop facial dysmorphism as a consequence of AFS compared to adults, usually in the form of proptosis [7–8]. This study aims to evaluate the different features of AFS in the pediatric population, to facilitate its early detection and eventually prevent its progression into intraorbital and intracranial complications.
METHOD
This is a retrospective case series study that includes several pediatric patients diagnosed with AFS and managed at a tertiary referral hospital in Saudi Arabia. We reviewed the different clinical presentations, radiographic features and treatment of each clinical entity.
CASE SERIES
Case 1 (unilateral)
A 12-year-old female presented with unilateral left-sided nasal discharge and progressive nasal obstruction. Computed tomography (CT) scan of the paranasal sinuses (Fig. 1) confirmed the diagnosis of AFS. Functional Endoscopic Sinus Surgery (FESS) was performed to clean the sinuses from polyps, mucin and fungal debris, foll owed by medical treatment. The patient remained symptom-free for 3 years follow-up.
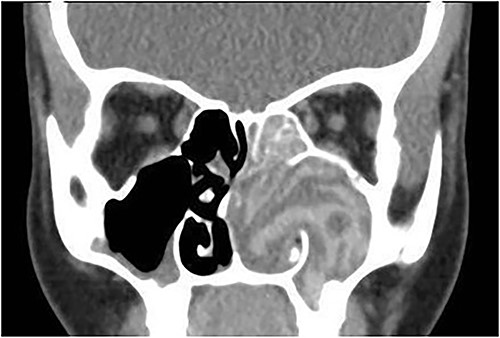
Coronal CT paranasal sinuses shows unilateral heterogeneous opacity of the obliterated left maxillary and ethmoid sinuses with extension through the widened remodeled ostiomeatal complex obliterating the left nasal cavity.
Case 2 (unilateral with proptosis)
A 13-year-old male presented with progressive right-sided nasal obstruction followed by slowly progressive right eye proptosis noticed by parents for 1 year duration. CT scan of the paranasal sinuses (Fig. 2) confirmed the diagnosis of AFS. The child underwent FESS and received medical treatments post-operatively. The patient remained symptom-free for 4 years follow-up.
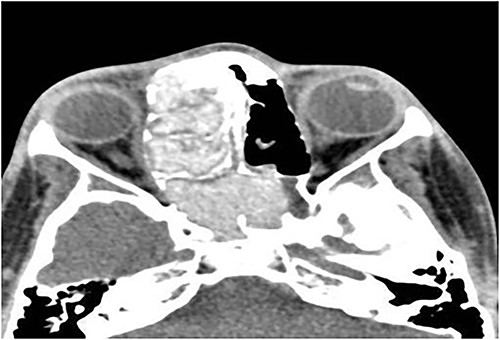
Axial CT paranasal sinuses shows the right ethmoid and sphenoid sinuses with complete obliteration and hyperdense (metallic) contents. It is associated with expansion of the ethmoid sinuses and lateral displacement of the right lamina papyracea causing right eye proptosis.
Case 3 (bilateral)
A 14-year-old male presented with a history of bilateral nasal obstruction for 3 years associated with snoring and postnasal discharge. Endoscopic examination showed bilateral nasal polyposis. CT scan of the paranasal sinuses (Fig. 3) & FESS, via which the sinuses were found full of polyps, mucin and fungal debris, confirmed the diagnosis of bilateral AFS. The patient continued on postoperative medical treatment and remained symptoms-free for 2 years follow-up.
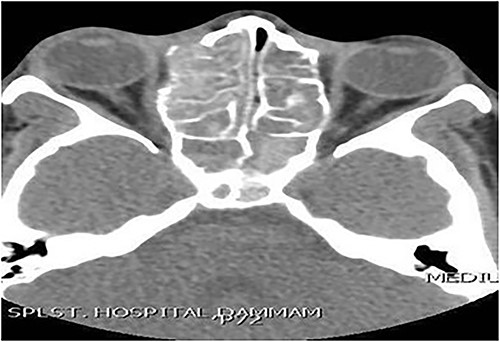
Axial CT paranasal sinuses shows heterogeneous opacities filling the ethmoid and sphenoid sinuses bilaterally with hyperdense contents. The ethmoid sinus shows expansion with partial dehiscence of the right lamina papyracea.
Case 4 (alternating)
A 8-year-old female with known bronchial asthma presented with right nasal obstruction & mild right eye proptosis for 9 months. Endoscopic nasal examination showed multiple right nasal polyps. CT scan of the paranasal sinuses (Fig. 4) and FESS confirmed the diagnosis of right-sided allergic fungal pansinusitis. After 3 years, she presented with left-sided nasal obstruction & discharge. Upon performing the endoscopic nasal examination, multiple nasal polyps were found in the left nose. CT scan of the paranasal sinuses (Fig. 5) confirmed the diagnosis of left-sided allergic fungal pansinusitis, but the operated right-sided sinuses were normal. The child remained symptoms-free for 5 years follow-up.
Coronal CT paranasal sinuses shows heterogeneous opacities filling the right frontal sinus with extension through the right frontal recess. It involves the ethmoid sinus with expansion. The right lamina papyracea shows lateral displacement with partial dehiscence and nasal polyposis.
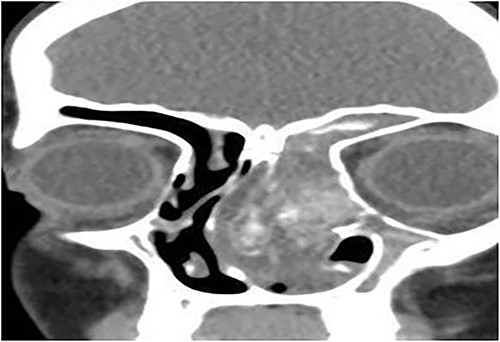
Coronal CT paranasal sinuses shows heterogeneous opacities filling the left frontal sinus with expansion of the left frontal recess & bone expansion and extension into the nasal cavity associated with nasal polyposis, resulting in near obliteration of the nasal cavity and deviation of the nasal septum to the right. Left maxillary antrum is involved. The right operated side is free of disease.
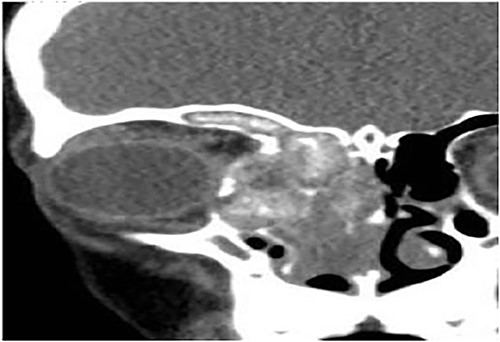
Case 5 (isolated sphenoid)
A 14-year-old male presented with a history of headache and postnasal discharge. CT scan of the paranasal sinuses (Fig. 6) and FESS confirmed the diagnosis of isolated sphenoid sinus AFS, as mucin and fungal debris were filling the sphenoid sinus only. The patient continued on postoperative medical treatment and remained symptom-free for 2 years follow-up.
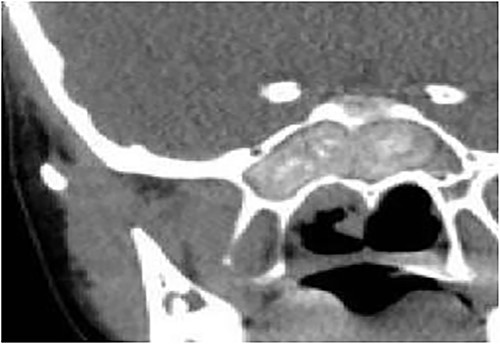
Coronal CT paranasal sinuses shows complete heterogeneous opacification of the sphenoid sinus with hyperdense contents and hypodense mucosa. No bone erosion or dehiscence were noted.
Case 6 (extensive into intraorbital and intracranial structures)
A 15-years-old male presented with bilateral nasal obstruction for a long time associated with nasal discharge, allergic nasal symptoms and headache. Endoscopic nasal examination revealed bilateral extensive nasal polyposis and mucin. CT scan (Fig. 7) and magnetic resonance imaging (MRI) scan (Fig. 8) of the paranasal sinuses confirmed the diagnosis of extensive bilateral allergic fungal pansinusitis with intraorbital and intracranial extradural extension. The patient underwent FESS, and all the polyps, mucin and fungal debris were removed from the sinuses. The patient continued on medical treatment and remained symptoms-free for 5 years follow-up.
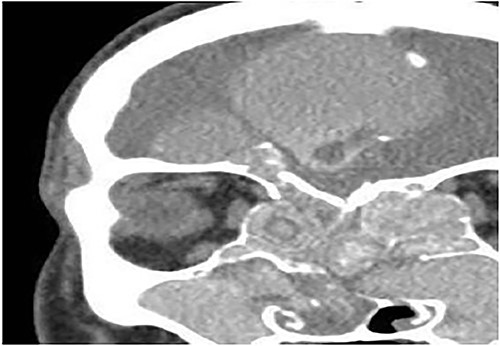
Coronal CT paranasal sinuses shows heterogeneous opacities of the ethmoid and maxillary sinuses bilaterally. It is associated with complete opacification and extension through the widened ostiomeatal complex, obliterating the nasal cavities with polyposis. The ethmoid sinuses show expansion with lateral displacement and partial dehiscence of the lamina papyracea bilaterally. The roof of the right ethmoid sinus shows dehiscence with intracranial extension.
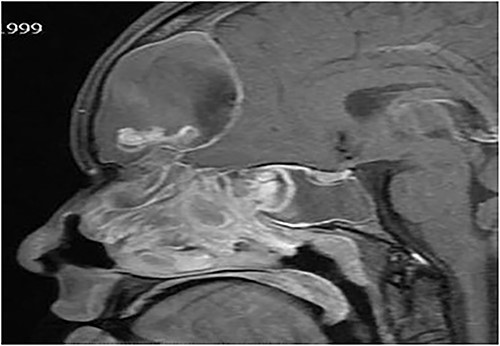
Sagittal T1 post contrast MRI paranasal sinuses shows expansion of the frontal sinus and anterior ethmoid with intracranial extension limited by the dura. The sphenoid sinus shows complete opacification and expansion displacing the pituitary fossa superiorly. The nasal cavity is obliterated by nasal polyposis.
DISCUSSION
In 1989, the term AFS was first introduced by Robson et al. [9]. They identified several fungi causing similar clinical features. The most common fungi responsible for AFS are Dematiaceous fungi, including Curvularia lunata, Bipolaris spicifera, Exserohilum rostratum, Alternaria, Drechslera, Helminthosporium, Chrysosporium, Rhizopus and Fusarium [10–14]. They account for ⁓84% of all cases, followed by Aspergillus species (13%) and other rare species (<1%) [15]. The clinical symptoms of patients with AFS are similar to those with chronic sinusitis, including rhinorrhea, nasal obstruction, headache and postnasal discharge. However, the presence of pain is uncommon in patients with AFS, and thus when present, it might suggest the synchronous presence of bacterial rhinosinusitis. Up to now, there are only a few articles that have described the course and features of AFS in the pediatric population [5, 11, 15, 21, 22]. The presenting symptoms and clinical course of AFS in children differ from that in adults. Moreover, AFS in children tends to be more severe, possibly because of their incompletely developed facial skeletons, placing them at risk of aggressive bone expansion, regional extension and erosion, eventually resulting in complications like telecanthus, proptosis, malar flattening, ophthalmoplegia, a bulge in the medial canthus and nasal pyramid widening [5, 8, 22]. Upon endoscopy, nasal polyposis and allergic mucin can be detected. The latter feature is reflected macroscopically in the form of highly viscous, tenacious, thick mucus [15]. Histologically, allergic mucin shows Charcot–Leyden crystals and fungal hyphae within sheets of eosinophils (Fig. 9). Pediatric patients with AFS have radiographic features similar to those in adult patients. CT scans show soft tissue mass with heterogeneous density in the sinuses with patches of calcifications inside [8]. That is usually associated with thinning of the bony wall of the affected sinuses, secondary to the direct progression of the disease [22]. Using MRI, a low T1-weighted signal and signal void on T2-weighted sequences are typical findings. The latter signal gives the impression that the disorder disappears on imaging. Moreover, the diagnostics of patients suspected to have AFS may include total serum immunoglobulin E (IgE) level, total eosinophil count, fungal antigen-specific immunoglobulin G, antigen-specific IgE for both fungal and other inhalants, microscopic evaluation, fungal culture of allergic mucin evacuated intraoperatively and precipitating antibodies [16–17]. A broad consensus on a proper treatment plan for AFS has not been reached yet [18], and both medical and surgical procedures are used. Long-term medical therapy includes topical or oral corticosteroids, immunotherapy and antifungal agents. The administration of steroids helps reduce mucosal edema, polyps and inflammation [15]. Meanwhile, the use of antifungal agents is controversial because it has shown variable results. Finally, many authors recommended the use of antibiotics in conjunction with steroids preoperatively to minimize the possibility of superadded postoperative bacterial sinusitis [15, 18, 19]. From a surgical point of view, there are no noticeable differences between adult and pediatric cases. Endoscopy, in which removal of fungal debris from the involved sinuses, establishment of normal mucociliary drainage pathways and debulking of diseased tissue are the primary goals, remains the standard therapeutic procedure for both adults & children. To avoid recurrence, the administration of nasal saline irrigation, nasal corticosteroid sprays and oral steroids in the postoperative period is imperative [16]. Nevertheless, systemic corticosteroids should gradually be tapered and stopped as soon as possible to avoid unwanted adverse effects, especially on the process of growth and bone development of the growing child [20–24]. Postoperative follow-up is mandatory for all patients to detect and treat early recurrences. In comparison to adults, children had a higher relapse rate ranging from 25 to 50% [8, 16, 22]. It has been hypothesized that this high rate of recurrence in the pediatric population is related to the risks associated with the long-term or repetitive use of systemic steroid therapy [15].
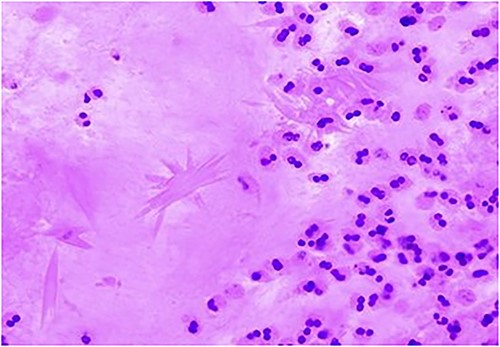
Charcot–Leyden crystal displayed using hematoxylin and eosin stain, indicating a mucin allergic in nature. It also shows non-invasive fungal hyphae & fungal elements with morphology consistent with Aspergillus species within the nasal mucus as in Fig. 10.
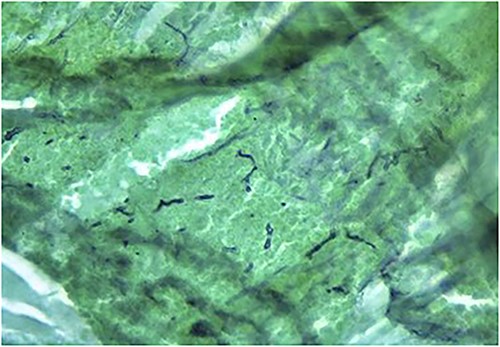
Fungal elements of a smear from paranasal sinus contents displayed using Grocott’s methenamine silver stain.
CONCLUSION
The clinical picture of pediatric AFS varies widely and includes unilateral, unilateral with proptosis, bilateral, alternating, isolated sphenoid and finally extensive into intraorbital and intracranial structures. Children with AFS present with different clinical features when compared with adults, requiring a high index of suspicion for evaluation and aggressive treatment without an unnecessary delay. Both medical and endoscopic surgical procedures are required for AFS treatment. Long-term clinical follow-up is essential to control recurrence and enhance prognosis.
ACKNOWLEDGMENTS
We thank the participants who have contributed to making this study possible.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
FUNDING
This study has not received any funding.
DATA AVAILABILITY STATEMENT
The study data is available from the corresponding author on reasonable request.
AUTHORS’ CONTRIBUTIONS
Dr. Fawatim Al Nahwi & Dr. Ali AlMomen conceptualized and designed the study, revised the manuscript and approved the final version to be submitted. Sarah Alkishi, Fadhel Almolani, Mohammed Al Ameer, Aziza Alzubaidi, Wejdan Suwayyid and Abdullah Alkhars collectively carried out the literature review and wrote the manuscript parts, including the introduction, methodology, discussion, conclusion, figures’ legends and references.
COMPLIANCE WITH ETHICAL STANDARDS
Written informed consent was obtained from the parents for the publication of this case series on behalf of the patient.



