-
PDF
- Split View
-
Views
-
Cite
Cite
Baijaeek Sain, Megan Blake, Kanchan Goyal, Harsimran Kaur, Kristina Robinson, Epstein–Barr virus-positive primary diffuse large B-cell lymphoma of the urinary bladder: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 3, March 2023, rjad111, https://doi.org/10.1093/jscr/rjad111
Close - Share Icon Share
Abstract
Primary non-Hodgkin’s lymphoma (NHL) of the urinary bladder is a rare event, with diffuse large B-cell lymphoma (DLBCL) being the most common form of NHL and urinary bladder lymphoma. It is an aggressive tumour with a poor prognosis if not recognised and treated early. The diagnosis is supported by radiological imaging and confirmed by histology, which shows the characteristic morphology of this lesion with further immunohistochemical analysis. Here we present a case of Epstein–Barr virus-positive DLBCL confirmed by an immunohistochemistry panel, along with a brief review of the literature focusing on diagnosis, treatment and outcome of this rare tumour.
INTRODUCTION
Lymphoma of the urinary bladder is a rare event. Primary non-Hodgkin’s lymphoma (NHL) of the urinary bladder accounts for 0.2% of extranodal NHL. The majority of bladder cancers arise from the urothelium, such as transitional cell and squamous cell carcinomas of the bladder, while lymphomas do not. It accounts for 5% of non-urothelial tumours in the urothelial tract and tends to affect more women than men [1–3]. Advanced age, male gender and lack of surgical resection or chemotherapy are indicators of poor prognosis. The clinical presentation and radiological findings are often non-specific, with symptoms such as haematuria, dysuria, frequent urination, nocturia and abdominal and back pain. Therefore, biopsy-proven bladder lymphomas with morphology and immunohistochemical analysis are often required for diagnosis [4].
CASE PRESENTATION
A woman in her 80s presented to the surgical emergency complaining of severe abdominal pain (grade 8/10) associated with constipation for 3 days and fever for 1 day associated with vomiting. She also had history of urinary incontinence and decreased urine output, worsening low back pain, unintentional weight loss of half a stone and unsteady feet with a history of falls. There was history of weakness in both lower limbs with generalised weakness and numbness in the right leg. There was no history of bloody stools or any recent changes in bowel habits, no haematuria and loss of appetite, and any lumps or bumps in the body. She had a history of transverse myelitis and essential hypertension. She underwent an appendectomy early in life. Her medications included oral corticosteroids, amlodipine, amitriptyline and bendroflumethiazide. She had no known allergies. Her family history was negative for any bowel or bladder or other medical condition. The patient lived alone and did all her daily activities independently.
On examination, she was febrile with NEWS2 Score = 01 (Temp = 38.5 degree centigrade). She appeared systemically well. There was no pallor, lymphadenopathy, pedal oedema or clubbing. The patient was haemodynamically stable with a blood pressure of 120/70 mmHg, neither tachycardic nor tachypnoeic; oxygen saturation was 97% on room air. On systemic examination, abdomen was distended with lower abdominal tenderness and a full bladder was noted. Per rectal examination revealed faecal load with a mildly reduced anal tone with no hard impacted stool or mass, ballooning or any blood and mucus. A lower limb neurological examination showed normal tone, 5/5 power in lower limb movements except 4/5 bilaterally for hip flexion, preserved light touch and proprioception bilaterally in lower limbs. Reflexes were elicited in upper limbs but the knee jerk and ankle jerk were not elicited. Plantar reflexes were down-going. Cardiorespiratory examination showed no abnormalities.
INVESTIGATIONS
Based on her clinical presentation, an urgent magnetic resonance imaging (MRI) of whole spine scan was performed, which revealed severe bilateral hydroureteronephrosis. Scout imaging showed irregular bladder wall thickening and moderate canal stenosis at L4/L5 with no evidence of cauda equina syndrome. Computed tomography (CT) with contrast medium was then performed for the abdomen and pelvis together with KUB. It revealed moderate to severe bilateral hydroureteronephrosis down to the urinary bladder with a lesion resembling like a possible posterior wall urinary bladder tumour (Fig. 1). There was no faecal impaction with mild ascites and no compression of the posterior wall tumour on the bowel. No evidence of perforation was present.
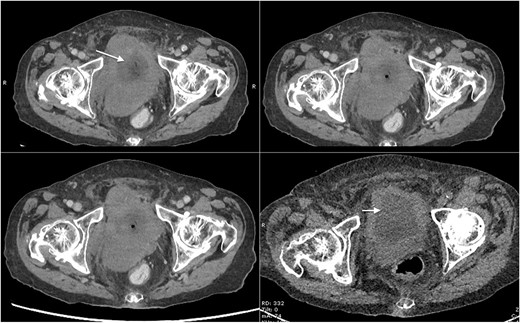
Axial CT scan of abdomen and pelvis with contrast showing mass lesion in the bladder (marked with arrows).
TREATMENT
After resuscitation, initial symptomatic treatment was done followed by catheterization, which subsequently became difficult because of obstruction in the ureter. The patient underwent a rigid cystoscopy with trans-urethral resection of bladder tumour (TURBT) and a left double-J stent was inserted. Bladder biopsies were obtained and sent for histopathological examination (Fig. 2). The biopsy showed an Epstein–Barr virus (EBV)-positive diffuse large B-cell lymphoma (DLBCL) with a non-germinal centre immunophenotype. In-situ hybridization for EBV-encoded RNA (EBER) cells were diffusely present (Fig. 3). Immunohistochemistry revealed the large atypical lymphoid cells showing CD45+, CD20+, CD30±, CD10−, BCL6+, MUM1+, BCL-2+, MYC− (<40% nuclei staining), CD5−, CD23−, cyclin D1− and TdT−. Plentiful T lymphocytes were seen in the background highlighted by CD3 and CD5. No follicular dendritic cell mesh works were present with CD21 or CD23. Ki-67 showed increased proliferation in the large lymphoid cells ~70–80% (Fig. 4). Based on the biopsy and immunohistochemistry results, this was diagnosed as an EBV-positive primary DLBCL. MDT discussion of the patient took place and she was recommended for supportive and palliative care team.
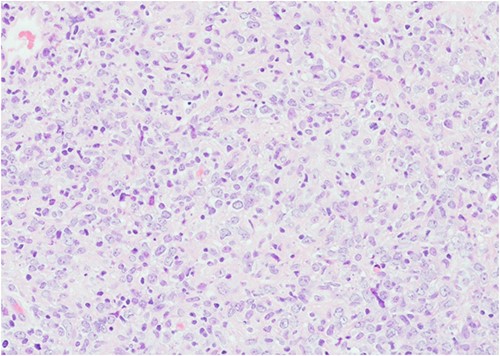
Histopathology image of urinary bladder showing extensive tissue necrosis and presence of tumour in the biopsy with no background normal bladder tissue (stained by H&E, power of magnification at ×200).
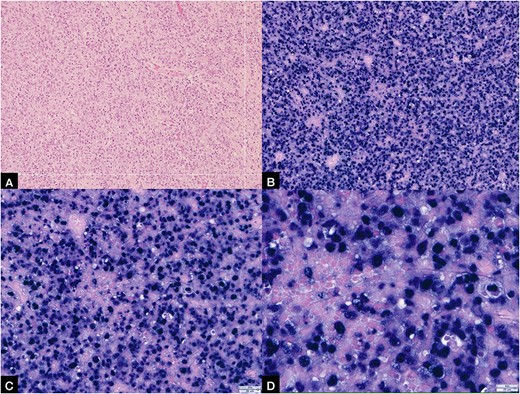
(A) Showing histopathology image of urinary bladder showing extensive tissue necrosis and presence of tumour in the biopsy with no background normal bladder tissue (stained by H&E, power of magnification at ×200); (B, C, D) showing EBV-positive EBER ISH-positive bladder tumour at ×10, ×20 and ×40 power of magnification, respectively.
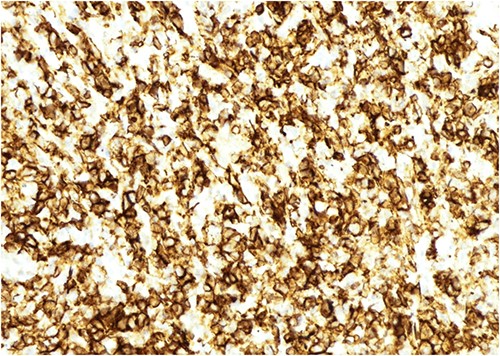
Figure showing immunohistochemistry slide positive for CD-20 (power off magnification at ×200).
OUTCOME AND FOLLOW-UP
Following the rigid cystoscopy with TURBT and left double-J stent insertion, the patient recovered well. Her blood results 1 day post-operation, in comparison to the days leading up to the operation, are as demonstrated in Table 1.
Table showing renal function investigation panel before and after the surgery
| Day wise investigations . | eGFR (ml/min/1.73 m2) . | Creatinine (umol/L) . | Urea (mmol/L) . |
|---|---|---|---|
| Days prior to operation | 26 | 162 | 15.8 |
| 24 | 173 | 15.8 | |
| 26 | 162 | 15.3 | |
| 25 | 167 | 16.4 | |
| 1 day post-operation | 41 | 110 | 9.8 |
| Day wise investigations . | eGFR (ml/min/1.73 m2) . | Creatinine (umol/L) . | Urea (mmol/L) . |
|---|---|---|---|
| Days prior to operation | 26 | 162 | 15.8 |
| 24 | 173 | 15.8 | |
| 26 | 162 | 15.3 | |
| 25 | 167 | 16.4 | |
| 1 day post-operation | 41 | 110 | 9.8 |
Table showing renal function investigation panel before and after the surgery
| Day wise investigations . | eGFR (ml/min/1.73 m2) . | Creatinine (umol/L) . | Urea (mmol/L) . |
|---|---|---|---|
| Days prior to operation | 26 | 162 | 15.8 |
| 24 | 173 | 15.8 | |
| 26 | 162 | 15.3 | |
| 25 | 167 | 16.4 | |
| 1 day post-operation | 41 | 110 | 9.8 |
| Day wise investigations . | eGFR (ml/min/1.73 m2) . | Creatinine (umol/L) . | Urea (mmol/L) . |
|---|---|---|---|
| Days prior to operation | 26 | 162 | 15.8 |
| 24 | 173 | 15.8 | |
| 26 | 162 | 15.3 | |
| 25 | 167 | 16.4 | |
| 1 day post-operation | 41 | 110 | 9.8 |
The patient was referred to a haematology clinic and urinary continence management support. She was stepped-down to a non-acute hospital closer to her home, and following the MDT decision and a discussion with the patient and her family, she was referred to the palliative care team where she is currently under end-of-life care.
DISCUSSION
NHL can develop in tissues other than the lymph nodes and even in sites where no lymphoid tissue is typically found. These are known as primary extranodal lymphomas, which occur in about a quarter of all lymphomas [1]. Commonly affected are the gastrointestinal tract, head and neck, skin, central nervous system, bones, testicles, breast and thyroid [2]. Primary lymphomas of the urinary bladder are extremely rare, accounting for 0.2% of all bladder cancers. In this rare subgroup, the majority of bladder lymphomas are primary NHL [3]. Lymphomas of the urinary bladder are more common in women than men, with women being 6.5 times more likely to have primary bladder lymphoma than men, with a mean age of 64 years (range 20–85 years) [4–5]. DLBCL is the most common form of NHL and the most common high-grade primary urinary bladder lymphoma (PUBL) [6]. MALT lymphoma is the most common low-grade PUBL and the most common type of bladder lymphoma according to the review by Simpson et al. However, high-grade DLBCL accounts for 20% of all primary bladder lymphomas [2]. In all, 40% of DLBCLs occur at extranodal sites. In addition, PUBL accounts for only 0.2% of NHL, which occurs at extranodal sites, and <1% of all urinary bladder neoplasms.
DLBCL is an aggressive lymphoma that carries a poor prognosis if not identified and treated early. Older age, male gender and lack of surgical resection or chemotherapy are poor prognostic factors [7]. EBV-positive DLBCL is less common, and carries a worse prognosis, than EBV-negative DLBCL [6].
The pathophysiology of primary NHL of the urinary bladder has not yet been fully identified or understood. Hypotheses include lymphoid proliferation with recurrent cystitis and cloacal embryonic remnant leading to the development of lymphoma [8]. Presentation is often non-specific but includes haematuria (most common), abdominal and back pain, and obstructive urinary symptoms such as dysuria, urinary frequency and nocturia, suprapubic pain and recurrent urinary tract infections [2, 9]. Patients are most likely at the time of diagnosis older than 60 years [6]. Because of these non-specific features coupled with the rarity of primary NHL of the urinary bladder, it can be very difficult to diagnose, and even more so to diagnose early [10].
Radiological investigation modalities include an ultrasound scan, where the tumour appears as a solid, homogeneous mass and CT scans, where findings include a soft-tissue dense mass that increases after intravenous the injection of contrast material [11]. Although ultrasound, CT and MRI scans can be useful in determining the size and location of a tumour in the urinary bladder, distinguishing between different types of malignancies requires cystoscopy and immunohistochemical evaluation of biopsies, which play an important role in diagnosis of the disease [6]. CD19, CD20 and CD21 immunohistochemical staining results for B-cell lymphomas are often positive. In addition, high-grade lymphomas are associated with CD3, CD20 and CD3, whereas low-grade lymphomas often test positive for CD20, CD21 and CD43 cell markers [12]. Bone marrow biopsy and PET scans are also used to assess other nodal or extranodal involvement in suspected primary bladder lymphoma to rule out systemic involvement if high-grade tumour is found [10].
Previous studies have used surgery, chemotherapy and radiation therapy, either individually or in combination, to treat patients with primary bladder lymphoma [5]. A detailed review of previously reported cases is included in Table 2. However, treatment for PUB DLBCL is primarily chemo-immunotherapy, specifically rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP). Zanelli et al. [6] state that no benefit of surgery has been identified in patients with PUB DLBCL. The importance of surgery is controversial given the likelihood of lymphoma spreading to other organs and surgical removal of the bladder affecting quality of life [5]. However, Simpson et al. [2] states that simple therapies (TURBT) are as effective as chemotherapy and should be used primarily for low-grade tumours and in combination with chemotherapy for high-grade tumours. Although radiotherapy can be used as an adjuvant after resection or in low-grade tumours, it cannot be considered a standard of care. Patient factors must also be considered when formulating a treatment plan, including age, physical capacity and physiological status, tumour stage and International Prognostic Index [10].
| Serial No. . | Age/sex . | Year of presentation . | Site . | Intervention . | Histopathological diagnosis . | Treatment . |
|---|---|---|---|---|---|---|
| 1. | 55-year male | 2004 [13] | Left lateral wall of Urinary bladder | USG + CT scan + histopathology + immunocytochemical stains | Diffuse large B-cell NHL of bladder | 4 cycles of chemotherapy with CHOP regimen |
| 2. | 80-year female | 2005 [14] | Right lateral wall | Ultrasound + cystoscopy + CT scan + transurethral biopsy | Non-Hodgkin large B-cell lymphoma of bladder | Chemotherapy |
| 3. | 67-year female | 2009 [15] | Right posterior and lateral wall of bladder | CT scan + biopsy + immunohistochemical study | EBV-positive polymorphous B-cell lymphoma of bladder | Palliative radiotherapy |
| 4. | 75-year female | 2009 [5] | Whole wall of bladder | Intravenous pyelography + CT scan + immunohistochemical study | Primary DLBCL of bladder—Stage IVA | 3 cycles of R-CHOP regimen |
| 5. | 66-year male | 2016 [16] | Dome of bladder | MRI + histopathology | EBV-positive DLBCL of bladder | Patient was on enzalutamide for treatment of prostate cancer. On cessation of enzalutamide, DLBCL of bladder regressed spontaneously |
| 6. | 40-year male | 2020 [10] | Bladder wall | Ultrasound + CT scan + histology + immunohistochemical studies | Primary B-cell lymphoma of the bladder | 6 cycles of chemotherapy with R-CHOP regimen + radiotherapy |
| Serial No. . | Age/sex . | Year of presentation . | Site . | Intervention . | Histopathological diagnosis . | Treatment . |
|---|---|---|---|---|---|---|
| 1. | 55-year male | 2004 [13] | Left lateral wall of Urinary bladder | USG + CT scan + histopathology + immunocytochemical stains | Diffuse large B-cell NHL of bladder | 4 cycles of chemotherapy with CHOP regimen |
| 2. | 80-year female | 2005 [14] | Right lateral wall | Ultrasound + cystoscopy + CT scan + transurethral biopsy | Non-Hodgkin large B-cell lymphoma of bladder | Chemotherapy |
| 3. | 67-year female | 2009 [15] | Right posterior and lateral wall of bladder | CT scan + biopsy + immunohistochemical study | EBV-positive polymorphous B-cell lymphoma of bladder | Palliative radiotherapy |
| 4. | 75-year female | 2009 [5] | Whole wall of bladder | Intravenous pyelography + CT scan + immunohistochemical study | Primary DLBCL of bladder—Stage IVA | 3 cycles of R-CHOP regimen |
| 5. | 66-year male | 2016 [16] | Dome of bladder | MRI + histopathology | EBV-positive DLBCL of bladder | Patient was on enzalutamide for treatment of prostate cancer. On cessation of enzalutamide, DLBCL of bladder regressed spontaneously |
| 6. | 40-year male | 2020 [10] | Bladder wall | Ultrasound + CT scan + histology + immunohistochemical studies | Primary B-cell lymphoma of the bladder | 6 cycles of chemotherapy with R-CHOP regimen + radiotherapy |
| Serial No. . | Age/sex . | Year of presentation . | Site . | Intervention . | Histopathological diagnosis . | Treatment . |
|---|---|---|---|---|---|---|
| 1. | 55-year male | 2004 [13] | Left lateral wall of Urinary bladder | USG + CT scan + histopathology + immunocytochemical stains | Diffuse large B-cell NHL of bladder | 4 cycles of chemotherapy with CHOP regimen |
| 2. | 80-year female | 2005 [14] | Right lateral wall | Ultrasound + cystoscopy + CT scan + transurethral biopsy | Non-Hodgkin large B-cell lymphoma of bladder | Chemotherapy |
| 3. | 67-year female | 2009 [15] | Right posterior and lateral wall of bladder | CT scan + biopsy + immunohistochemical study | EBV-positive polymorphous B-cell lymphoma of bladder | Palliative radiotherapy |
| 4. | 75-year female | 2009 [5] | Whole wall of bladder | Intravenous pyelography + CT scan + immunohistochemical study | Primary DLBCL of bladder—Stage IVA | 3 cycles of R-CHOP regimen |
| 5. | 66-year male | 2016 [16] | Dome of bladder | MRI + histopathology | EBV-positive DLBCL of bladder | Patient was on enzalutamide for treatment of prostate cancer. On cessation of enzalutamide, DLBCL of bladder regressed spontaneously |
| 6. | 40-year male | 2020 [10] | Bladder wall | Ultrasound + CT scan + histology + immunohistochemical studies | Primary B-cell lymphoma of the bladder | 6 cycles of chemotherapy with R-CHOP regimen + radiotherapy |
| Serial No. . | Age/sex . | Year of presentation . | Site . | Intervention . | Histopathological diagnosis . | Treatment . |
|---|---|---|---|---|---|---|
| 1. | 55-year male | 2004 [13] | Left lateral wall of Urinary bladder | USG + CT scan + histopathology + immunocytochemical stains | Diffuse large B-cell NHL of bladder | 4 cycles of chemotherapy with CHOP regimen |
| 2. | 80-year female | 2005 [14] | Right lateral wall | Ultrasound + cystoscopy + CT scan + transurethral biopsy | Non-Hodgkin large B-cell lymphoma of bladder | Chemotherapy |
| 3. | 67-year female | 2009 [15] | Right posterior and lateral wall of bladder | CT scan + biopsy + immunohistochemical study | EBV-positive polymorphous B-cell lymphoma of bladder | Palliative radiotherapy |
| 4. | 75-year female | 2009 [5] | Whole wall of bladder | Intravenous pyelography + CT scan + immunohistochemical study | Primary DLBCL of bladder—Stage IVA | 3 cycles of R-CHOP regimen |
| 5. | 66-year male | 2016 [16] | Dome of bladder | MRI + histopathology | EBV-positive DLBCL of bladder | Patient was on enzalutamide for treatment of prostate cancer. On cessation of enzalutamide, DLBCL of bladder regressed spontaneously |
| 6. | 40-year male | 2020 [10] | Bladder wall | Ultrasound + CT scan + histology + immunohistochemical studies | Primary B-cell lymphoma of the bladder | 6 cycles of chemotherapy with R-CHOP regimen + radiotherapy |
CONCLUSION
Primary DLBCL of the urinary bladder is a rare disease. The prognosis for DLBCL, an aggressive lymphoma, is bleak if not recognised and treated quickly. Compared with EBV-negative DLBCL, EBV-positive DLBCL is rarer and has a poorer prognosis. Early detection and diagnosis of the disease is crucial to start treatment as soon as possible. Since the rarity and the non-specific appearance of the disease represent a diagnostic challenge, the immunohistochemical analysis is crucial for the diagnosis in addition to the imaging. Symptom management is essential to patient management and can have a significant impact on their quality of life. While previous research has used surgery, chemotherapy and radiotherapy either individually or in combination, chemo-immunotherapy, specifically rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone, is the main treatment modality for PUB DLBCL (R-CHOP). However, when a patient is able to refuse treatment, patient autonomy must be preserved and not neglected.
LEARNING POINTS
Early recognition and diagnosis of this rare disease is essential to be able to begin treatment as soon as possible.
Immunohistochemical evaluation is essential in addition to imaging to reach a confirmatory diagnosis with EBER cells in order to prove EBV-positive DLBCL.
Patient autonomy must be respected and if the patient has capacity and declines treatment, this cannot be ignored.
Symptom management is essential in the management of the patient and can have a significant impact on their quality of life.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



