-
PDF
- Split View
-
Views
-
Cite
Cite
Alix Wells, Ramesh Lokanathan, Mitchell Webb, Jessica J Lie, In situ open repair of renal artery aneurysm in 43-year-old with solitary kidney: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 12, December 2023, rjad702, https://doi.org/10.1093/jscr/rjad702
Close - Share Icon Share
Abstract
Renal artery aneurysms are rare. Indications for management include size > 3 cm, female gender within childbearing age, pain, hematuria, medically refractory hypertension, thromboembolism, dissection, and rupture. Management options include endovascular, open repair, and ex vivo approaches. A 43-year-old female with a history of polycystic kidney disease, solitary kidney, and uncontrolled hypertension was found to have a proximal large renal aneurysm on imaging. The patient underwent an in situ open aneurysm resection, temporary shunt insertion, and patch repair with good postoperative outcomes. Whereas previous studies showed the success of ex vivo repair and autotransplantation in large aneurysms with solitary kidneys, our case demonstrated that in situ open repair and patch with the use of a temporary shunt is a feasible and effective option. In a patient with a solitary kidney and large proximal renal artery aneurysm, an in situ open approach and patch repair with shunt insertion should be considered.
Introduction
Renal artery aneurysms (RAAs) were first described in the literature by Rouppe [1]. Such aneurysms are quite rare and are estimated to occur in 0.1% of the population [2].
Predisposing factors often associated with RAAs include female sex, fibromuscular dysplasia, atherosclerotic disease, and the sixth decade of life [3, 4].
Once the diagnosis has been made, the Society of Vascular Surgeons recommends treatment for size > 3 cm as well as female gender within childbearing age, symptoms such as pain and hematuria, medically refractory hypertension, thromboembolism, dissection and rupture [4]. Management options include open repair procedures such as aneurysm resection with primary closure, ex vivo repair, and aneurysm resection with patch angioplasty as well as endovascular approaches such as stent graft and coil embolization [5]. To date, there are no studies of in situ repair in the context of RAA in a solitary kidney.
Presented here is the case of a 43-year-old female with a solitary kidney and large RAA who underwent success in vivo repair with patch and temporary shunt.
Case report
A 43-year-old female with a history of polycystic kidney disease, hypertension, menorrhagia, a 3-pack-year smoking history, and fatty liver disease was being followed by obstetrics and gynecology and referred for an abdominal ultrasound during investigations for menorrhagia and iron deficiency. She also had uncontrolled hypertension and occasional flank pain. The patient had a family history of brain aneurysms and had one aunt with a renal aneurysm.
Findings from the abdominal ultrasound demonstrated a normal-sized right kidney with a 3.5 × 3.2 × 3.4 cm structure at the hilum which had turbulent pulsatile flow, consistent with a renal aneurysm. No distinct left kidney could be visualized.
Computed Tomography (CT) renal angiogram was consistent with prior imaging, demonstrating a 3.8 cm right renal aneurysm, multicystic dysplastic appearance to the left kidney with no functional left renal tissue (Fig. 1). Hence, vascular surgery consultation was advised.
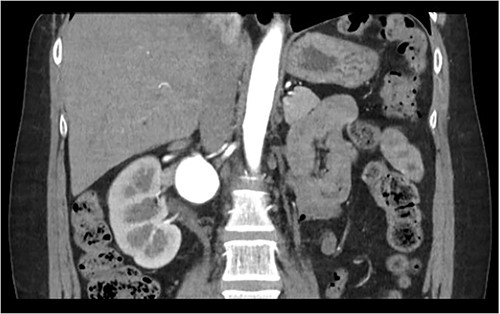
The patient was seen by a rheumatologist for assessment of a genetic cause of a solitary renal aneurysm such as fibromuscular dysplasia or vascular Ehlers Danlos. However, given her clinical presentation and imaging findings, this was not believed to be the case. During this initial consultation, the patient’s physical examination revealed blood pressure of 179/87 in the right arm and 173/124 in the left arm, reflecting uncontrolled hypertension. At the time of consultation, the patient was on Ramipril 5 mg PO once daily and was subsequently increased to Ramipril 5 mg PO BID afterwards. The patient was taken to the operating room for an open repair and resection of the right renal aneurysm with patch angioplasty. In the operating room, a laparotomy was performed, and the saccular aneurysm wall was resected and repaired with a bovine pericardial patch (Fig. 2). During the repair, an 8 mm Argyle shunt was inserted easily to maintain renal perfusion and prevent warm ischemia time. There was between 5 and 10 min of right renal artery ischemia during the procedure. The patch was closed and there were good Doppler signals distally. At the end of the procedure, the patient was making urine. There were no intraoperative complications.
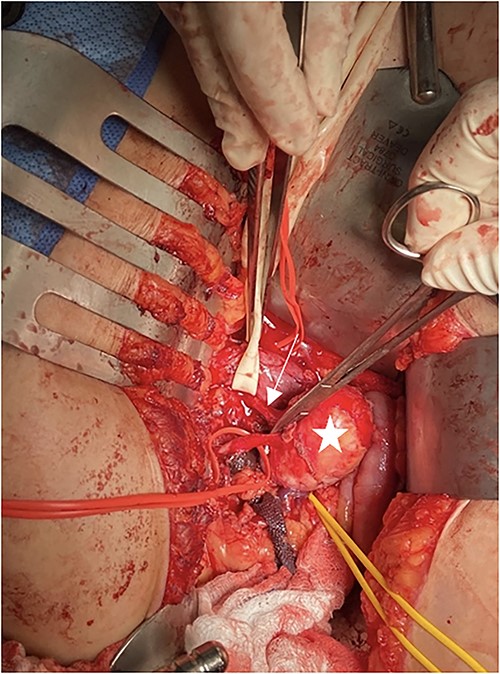
Intraoperative picture of RAA. Penrose is around the inferior vena cava, arrow shows the renal artery, star is on the RAA, and yellow vessel loop is around the renal vein.
Postoperatively, the patient’s course in the hospital was relatively unremarkable. Lab values of note during the patient’s stay were postoperative day (POD) 1: Hgb:82, Cr:160; POD 2: Hgb:72, Cr:135; POD 3: Hgb:75, Cr:111; POD 5: Hgb:88, Cr:88. The patient had an elevated blood pressure of 170/90 pre-op and post-op her BP ranged from 121/72 to 167/90, which was managed with only one dose of amlodipine. The patient was discharged on POD 5. Postoperative CT at 4 months demonstrated a patent repair and a well-perfused right kidney.
Discussion
Our patient is a 43-year-old woman with solitary kidney from polycystic kidney disease and uncontrolled hypertension, who was found to have a 3.8 cm RAA. Preoperative diagnosis enabled a successful repair of the aneurysm via resection and patch angioplasty.
These patients are often asymptomatic. However, if patients do have signs and symptoms, they include difficult to control hypertension, hematuria, flank pain, and abdominal pain [5]. The preferred diagnostic modality is CT-angiogram [6]. Most RAAs are solitary in nature, right-sided, saccular and on average between 1.3 and 3.8 cm [4, 5]. RAAs occurring in solitary kidneys, however, are quite limited within the literature. Our patient was presented with uncontrolled hypertension and occasional flank pain and was found to have a RAA on abdominal U/S, confirmed by CT-angiogram.
Indications for repair include size > 3 cm, female gender within childbearing age, symptoms such as pain and hematuria, medically refractory hypertension, thromboembolism, dissection, and rupture [4]. Management options include classical in situ techniques such as patch angioplasty, primary reanastomosis, primary angioplastic closure with or without branch reimplantation, interposition bypass, aorto-renal bypass, plication of small aneurysms, and splanchno-renal bypass [4]. Abreu et al. describe the intracorporeal robotic repair of RAA in solitary kidney via primary angioplastic closure with good postoperative outcomes. To date, there are no other case reports or series of in situ repair in the context of RAA in a solitary kidney [7].
Ex vivo repair with renal-renal bypass and cold preservation is typically reserved for more complex distal branch lesions [4, 8]. Desai et al. [9] and Palcau et al. [10] describe the management of RAAs in solitary kidney with ex vivo repair with autotransplantation. Both RAAs were distal and complex, hence good candidates for this approach. Endovascular options include stent graft exclusion and coil embolization for proximal and simple RAA [4]. We chose to pursue an in situ open approach given the aneurysm’s proximity to the branching of the renal artery, to avoid postembolization syndrome and the potential patency benefits of open repair over endovascular repair in this young patient. We did not feel that ex vivo offered extra benefit when compared to in vivo with a shunt.
Conclusion
We present to you the case of a 43-year-old asymptomatic female with uncontrolled hypertension found to have a large RAA in the context of a solitary kidney. Our patient, whose 4-month post-op CT demonstrated a patent repair and a well-perfused right kidney, suggests that an in situ open approach with aneurysm resection and subsequent patch repair is an option with good outcomes.
Author contributions
Study concept and design: Ramesh Lokanathan, Mitchell Webb, Jessica J. Lie. Acquisition, analysis, and interpretation of data: Alix Wells, Jessica J. Lie. Drafting of the manuscript: Alix Wells, Jessica J. Lie. Critical revision of the manuscript for important intellectual content: Alix Wells, Jessica J. Lie. Statistical analysis: n/a. Administrative, technical, or material support: Ramesh Lokanathan, Mitchell Webb, Jessica J. Lie. Study supervision: Ramesh Lokanathan, Jessica J. Lie.
Conflict of interest statement
None declared.
Funding
None declared.
Consent
Informed consent has been obtained and all identifying information is omitted.



