-
PDF
- Split View
-
Views
-
Cite
Cite
Osama A Alrodiman, Fahad A Alwadi, Mohammed Almahdi, Majed Pharaon, Low-grade sinonasal non-intestinal-type adenocarcinoma: a rare case report and literature review, Journal of Surgical Case Reports, Volume 2023, Issue 12, December 2023, rjad646, https://doi.org/10.1093/jscr/rjad646
Close - Share Icon Share
Abstract
Sinonasal non-intestinal-type adenocarcinoma is a rare but important differential diagnosis in patients presenting with recurrent, unexplained epistaxis. Low-grade types have a more favourable prognosis as opposed to the more aggressive high-grade. Symptoms include nasal obstruction and epistaxis that can last up to 5 years. We report a case of a rare low-grade sinonasal non-intestinal-type adenocarcinoma in a 43-year-old male who is frequently exposed to wood and dust particles. Endoscopy revealed right nasal mass occupying the entire nasal cavity as well as inferior turbinate hypertrophy and mass attached to the nasal septum on computed tomography. Biopsy confirmed the diagnosis and was classified as pT1NX with the presence of mitotic figures, which are more commonly present in the high-grade subtype.
Introduction
Nasal cavity and paranasal sinus cancers are relatively rare conditions, accounting for 3% of Head & Neck Cancers with a male predominance (male: female ratio of 1.8:1). The most frequently observed histological types are squamous cell carcinoma (51.6%) and adenocarcinoma (12.6%). The nasal cavity (43.9%) and maxillary sinus (35.9%) are the most common sites of occurrence [1].
Non-salivary types sinonasal adenocarcinoma has two main subtypes: intestinal and non-intestinal (n-ITAC). n-ITAC tumours originate from seromucinous glands and occur anywhere in the sinonasal tract. n-ITAC is further classified into high-grade and low-grade. High-grade n-ITAC are rare malignancies found in the maxillary sinuses with a poor prognosis (3-year survival rate of 30%) [2]. Rare low-grade n-ITAC primarily affects the nasal cavity, ethmoid sinuses, and maxillary sinuses. They feature uniform band cells forming papillary and glandular structures [3]. Symptoms include nasal obstruction and epistaxis for 2 months to 5 years [4], with an overall favourable prognosis (5-year survival rate reaching 85%) and are not associated with environmental carcinogens [2, 5].
We present a case of a 43-year-old male diagnosed with low-grade n-ITAC with mitotic figures.
Case presentation
A 43-year-old male presented to our clinic with intermittent right-sided epistaxis, nasal obstruction, and headache for 8 months. No history of post-nasal drip, visual changes, neck masses, trauma, illicit drug use, compressive symptoms, constitutional symptoms, or haematological disorders. Medical history includes diabetes mellitus, hypertension, dyslipidaemia, and non-ST elevation myocardial infarction with a prior percutaneous coronary intervention in 2018. The patient is previous smoker with a positive family history of lung cancer but no head and neck malignancies. Patient works in maintenance and is exposed to wood and dust.
On endoscopic assessment, the findings revealed the presence of bilateral anterior septal crustrations, as well as a right nasal mass that obstructed the nasal cavity, impeding the passage of the scope. The left nasal cavity and nasopharynx appeared clear and free from any abnormalities. No palpable neck masses or lymph nodes were detected. The oral cavity and bilateral ears exhibited normal characteristics, and the integrity of cranial nerves and facial sensation was intact.
Sinus computed tomography (CT) (Fig. 1a–c) revealed mild mucosal hypertrophy of the inferior turbinates and a non-enhancing mass lesion attached to the cartilaginous part of the nasal septum causing partial obstruction. Nasal endoscopic examination was performed under general anaesthesia for mass excision and biopsy. The mass was smooth, pedunculated, and arising from the septum. Pathology results indicated a diagnosis of sinonasal low-grade n-ITAC, classified as pT1 NX.
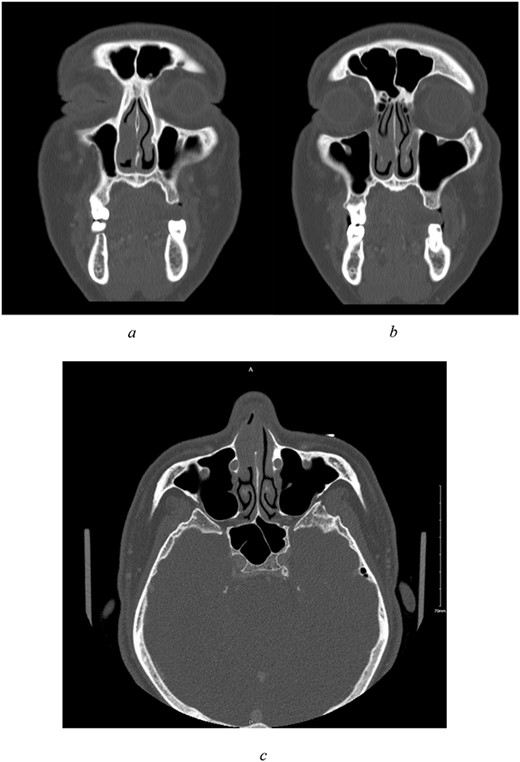
(a–c) Preoperative CT scan of paranasal sinuses with contrast. All imaged paranasal sinuses appears clear with no significant mucosal thickening or air-fluid level detected. Intact bony boundaries of the paranasal sinuses. (a, b) Coronal cut, showing clear paranasal sinuses, intact bony boundaries, and a non-enhancing soft tissue mass in the right anterior nasal cavity with poor demarcation to right inferior turbinate, attached to the cartilaginous part of nasal septum. (c) Axial cut.
On immunohistochemistry the tumour was positive for CK7 (OV-TL 12/20), S100 (polyclonal), Ki-67 (MIB-1) ~10% with focal area reaching up to 20%. The tumour was negative for CK20 (KS20), CDX2 (ERP2764Y), P63 (DAK-p63), collagen IV (CIV22), P40 (ZR8), CK5/6 (D5/16B4), Calponin (CLAP), TFF-1 (SPT24), and Vimentin (V9). Histologic examination reveals an unencapsulated glandular growth, composed of numerous back-to-back small glands and few dilated cystic spaces with no intervening stroma. The glands are lined by a single layer of non-ciliated, cuboidal to columnar cells with uniform, round nuclei, abundant eosinophilic cytoplasm, and occasional prominent nucleoli. Mitotic figures are counted at 3 per 10 high power fields (Fig. 2a and b). No necrosis seen. Tumour cells were diffusely positive for CK7 and weakly positive for S100 (Fig. 3a and b). Other markers such as CK20, CDX-2 (Fig. 3c), p63, CK5/6, Calponin, TTF-1, and vimentin were negative. Ki-67 (MIB 1 clone) proliferation index was estimated at 10% with focal areas reaching up to 20% (Fig. 3d).
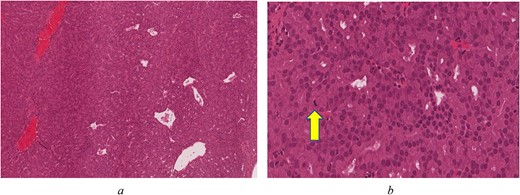
(a) Back-to-back glands with few dilated blood vessels and no intervening stroma (H&E*, original magnification ×80). (b) Compact glands with bland, monotonous, round nuclei. Arrow indicates a mitotic figure (H&E*, original magnification ×400) *Haematoxylin and Eosin.
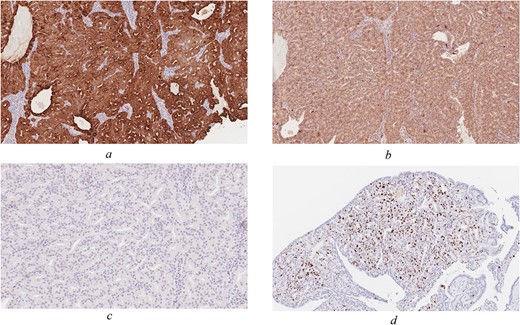
Immunohistochemical staining of the tumour (original magnification ×100). CK7 (a) and S-100 (b) are positive, whereas CDX-2 (c) is negative. (d) Ki-67 proliferation index focally reaches up to 20%.
Metastatic work-up showed no signs of lymphadenopathy or distant metastasis on head and neck, chest and abdomen/pelvis CT. Endoscopic wide local septectomy was then performed to achieve negative margins.
Patient shows significant symptom improvement and scheduled for nasal reconstruction because of aesthetic concerns.
Discussion
Low-grade n-ITAC account for 13% of sinonasal adenocarcinoma accounting for just 13% of cases [6]. Neoplasms of the sinonasal n-ITAC are uncommon and phenotypically diverse. Low-grade n-ITAC are typically papillary/tubular and constituted of cuboidal–columnar epithelial cells [7].
Patients with low-grade n-ITAC report persistent epistaxis, which can be misdiagnosed as nasal dryness. The exact aetiology of low-grade n-ITAC remains elusive. Despite this, our patient’s repeated exposure to wood and dust raises questions about risk factors [8]. Wilkerson et al. [9] also report a case of n-ITAC associated with previous radiation therapy for acute lymphoid leukaemia.
The distinction between low-grade and high-grade and n-ITAC is crucial given the distinct clinical behaviour, prognosis, as well as management. Histological characteristics of high-grade n-ITAC include solid growth patterns with sheets of cells, poorly defined and irregular glandular patterns, hyperchromatism, mild to evident nuclear pleomorphism, and a high mitotic rate [3]. According to the histology report, the biopsied tissue was positive for mitotic figures (counted at 3 per 10 high power fields). This is especially noteworthy given that mitotic figures are believed to be extremely rare in low-grade n-ITAC and more prevalent in the more severe high-grade subtype [6].
Because of their extreme heterogeneity, these tumours are difficult to identify and Immunohistochemistry is used to make the diagnosis, since the tumours frequently stain positive for CK7 and negative for CK20, CDX2, and villin, suggesting a respiratory-type profile as opposed to an intestinal-type profile, which was consistent with our patient.
Fortunately, as a result of its of low-grade origin, it has a favourable prognosis and may be treated surgically with rapid results as opposed to the high-grade origin. Endoscopic surgical resection is commonly performed throughout the literature, in accordance to a retrospective analysis of 17 patients who all underwent surgical excision alone. In all, 14 out of the 17 patients did not have local recurrence with a median of 60 months after diagnosis, and the remaining three patients required multiple surgical excisions with no evidence of subsequent disease progression [4]. In some cases in the literature, radiotherapy was used as the primary treatment, such as the case reported by Jain et al. [10], in which a 67-year-old patient with a low-grade n-ITAC nasopharyngeal mass partially responded to radiotherapy, but it subsequently progressed from low-grade to the more aggressive high-grade. Ultimately they resorted to an endoscopic endonasal surgical resection and chemotherapy given the aggressive nature [10].
Sinonasal malignancies do not often manifest symptoms until they reach a specific size and growth. Hence, a high level of suspicion is necessary to minimize diagnostic delays. In our case, a worrisome lesion was detected upon physical examination and radiographic imaging; therefore, prompt endoscopic nasal assessment and biopsy were done.
Conclusion
This put forward case demonstrates the need of keeping a high clinical suspicion for n-ITAC when presented with a history of chronic, unilateral epistaxis. The prognosis for low-grade n-ITAC is excellent, with full recovery following total surgical excision. Yet, this may be at the expense of aesthetic concerns.
Conflict of interest statement
None declared.
Funding
None declared.
Ethical approval
Our institution does not require ethical approval for case reports without patient identification and anonymity.
Consent
Consent to publish the case report was not obtained. This report contains no personally identifiable information that could lead to the patient’s identification.



