-
PDF
- Split View
-
Views
-
Cite
Cite
Yasnaia Bruneel, Christophe Ghysel, Caroline Van Holsbeke, Philippe Van Trappen, Fluorescence guidance during robotic resection of bladder wall endometriosis: case report and technique, Journal of Surgical Case Reports, Volume 2023, Issue 10, October 2023, rjad604, https://doi.org/10.1093/jscr/rjad604
Close - Share Icon Share
Abstract
The application of indocyanine green (ICG) has recently been reported to aid in the resection of endometriosis in the bladder wall and/or involving the ureters. A symptomatic 41-year-old patient with dysmenorrhea and pollakisuria was referred to our tertiary center. Imaging revealed a 1.5–2 cm intramural endometriotic nodule in the posterior bladder wall. She was planned for robotic resection of the endometriotic nodule, under ICG guidance, together with a hysterectomy. After placement of double-J ureteral stents and clamping the bladder, perforation of the bladder mucosa could be avoided whilst performing a circumferential resection of the nodule. By clamping the bladder catheter after instillation of ICG, both the bladder wall thickness and ureters could be visualized with near-infrared imaging during robotic resection of the endometriotic nodule and hysterectomy. With the surgical approach described here, endometriotic nodules/tissue can be removed precisely with enlarged vision at the robot console, safely, and completely without damaging adjacent tissues.
Introduction
Endometriosis is a chronic inflammatory disease characterized by the presence of endometrial like epithelium and/or stroma outside the uterine cavity. It is highly prevalent and it affects up to 10% of women within their reproductive years. It is often associated with severe symptoms including pain, such as dyspareunia and dyschezia, and infertility [1, 2]. To classify endometriosis, several classification systems have been developed, such as the revised American Society for Reproductive Medicine classification and the ENZIAN classification. [3, 4]. Following the guidelines of the European Society of Human Reproduction and Embryology, deep endometriosis can be defined as the involvement of endometrial-like tissue with a depth of >5 mm [2]. Deep endometriosis of the bladder is often associated with the involvement of the muscular layer of the bladder and/or ureteral ostia, and ureters. Urinary tract endometriosis is rare and occurs in 0.2–2.5% of all women with deep endometriosis [1]. Bladder endometriosis can be assessed by additional imaging, including ultrasonography, magnetic resonance imaging, and computed tomography (CT) [1, 5–8]. In bladder endometriosis surgery, dissection may begin in the healthy peritoneum adjacent to the endometriotic nodule, either paravesically or between the bladder and uterus. To enhance the intraoperative vision of the bladder and ureters, indocyanine green (ICG) fluorescence imaging, with near-infrared (NIR) imaging, can be applied to facilitate the dissection [9]. ICG is visualized only with NIR imaging, which can be found on the da Vinci Xi surgical system (Intuitive Surgical Inc), equipped with Firefly technology. When performing complex endometriosis surgery, it is important to eliminate all endometriotic lesions/tissue to prevent recurrence, whilst respecting the contours of the adjacent organs.
Here, we describe a robotic surgical approach (with Supplementary video) on the application of ICG for robotic resection of endometriosis in the posterior bladder wall, with visualizing the bladder wall thickness as well as the distal ureters.
Case presentation
A 41-year-old Caucasian G2P0A2 with symptoms of dysmenorrhea and pollakisuria, resistant to hormonal treatment, was referred to our tertiary center. Her medical history includes twice a miscarriage, operations at foot, knee, and arm, perianal abscess, and laparoscopy for endometriosis. She is an occasional smoker.
Expert transvaginal ultrasound scan was performed after referral with a CT scan to our tertiary center (Fig. 1). A 1.5–2 cm intramural endometriosis nodule was seen in the posterior bladder wall, not reaching the bladder mucosa (Fig. 2). On ultrasound scan, there was also evidence of adenomyosis of the uterus.
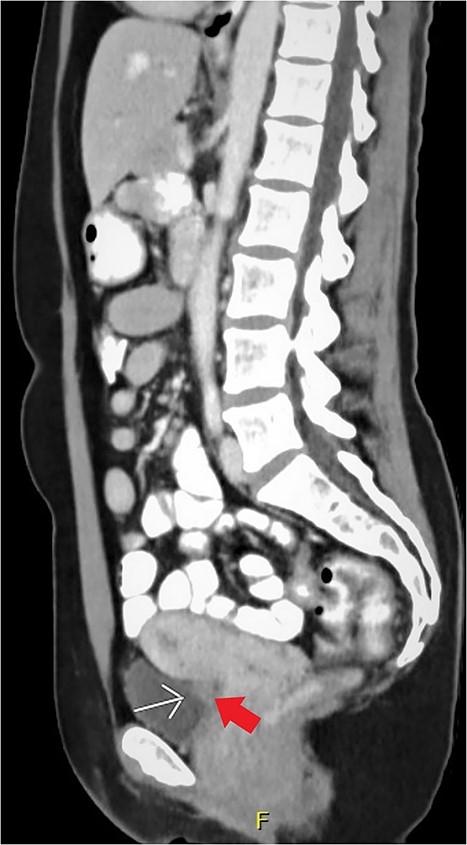
CT scan, sagittal view of the endometriosis nodule in the posterior bladder wall.
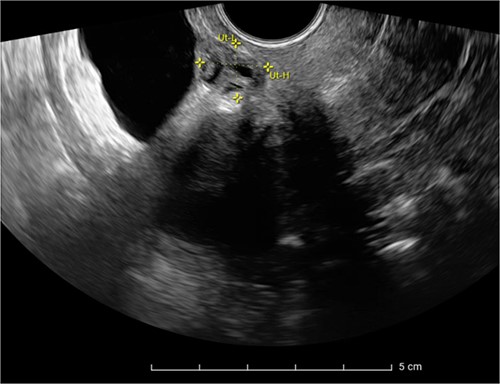
Ultrasound scan showing a heterogeneous endometriosis nodule in the posterior bladder wall not reaching the bladder mucosa.
The patient was planned for a robotic resection of the endometriosis nodule and hysterectomy by an experienced robotic gynecological surgeon. The patient was placed on a PinkPad (Kebomed Europe AG) in a lithotomy position. Cystoscopy was performed with placement of ureteral stents. Double-J stents were guided up to the kidneys. Instillation of 200 ml physiologic water with 2 cc of ICG (1.9 mg/cc) in an empty bladder, with subsequent clamping of the urinary catheter (Fig. 3). The da Vinci Xi robot platform (Intuitive Surgical Inc.) was used. Instruments used were: bipolar fenestrated forceps, unipolar scissor, Cadière forceps, and Vessel Sealer Extend. Circumferential incision, dissection and detachment of the endometriotic nodule from the posterior bladder wall was performed under guidance of NIR imaging and ICG, whilst the endometriosis nodule remained stuck on the uterine isthmus (Fig. 4; Supplementary Video). During the dissection, perforation of the bladder mucosa could be avoided, with transparency of the bladder mucosa by ICG. During the hysterectomy part, the bladder catheter was unclamped. The posterior bladder wall was repaired longitudinally. The muscular layers and serosa of the bladder were sutured with Monocryl 3/0 in a two-layer closure (Fig. 5). The bladder was filled with 200 cc of physiologic water with ICG, no bladder leakage nor clear transparency by ICG of the posterior bladder wall was seen. The total operative time was 120 min. The total blood loss was 25 mL. There were no perioperative complications. The length of the hospitalization was 48 hrs. Oxybutynin was given to prevent bladder spasms from the urinary catheter and/or stents. The Foley catheter was removed after 9 days, and the ureteral stents removed after 10 days. At the 2-week outpatient visit the patient was satisfied, and didn’t experience any pain nor urinary voiding problems.
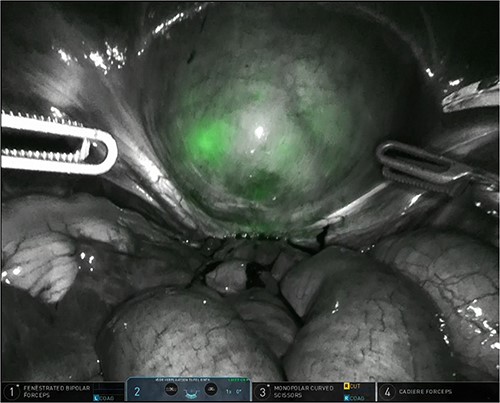
Bladder filled with physiologic water and ICG with subsequent clamping of the urinary catheter.

Robotic dissection of the endometriosis nodule between the isthmus of the uterus and bladder; lateral dissection with bilateral transparency of ICG of the thin posterior bladder wall.
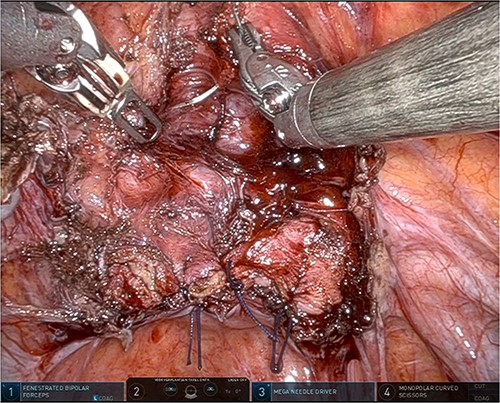
Suturing the posterior bladder wall in a two-layer closure, first the submucosa and muscular layer and second the muscular layer and serosa.
Discussion
The surgical approach presented here demonstrates the usefulness of ICG with NIR imaging for optimal visualization of bladder wall thickness as well as the ureters, when performing urinary tract endometriosis surgery, in particular bladder wall endometriosis.
When performing deep infiltrating endometriosis surgery, localization of the ureters and bladder margins can be difficult due to fibrotic tissue and adhesions [2]. Intraoperative ICG has been described previously to aid in the resection of endometriosis [9–11]. Here, we demonstrate a surgical robotic technique that allows easier identification of the bladder and ureters. In our case, ICG with physiologic water was administered into an empty bladder, prior to robotic docking, with subsequent clamping of the urinary catheter.
The current literature on ICG for visualizing the urinary tract is limited. Thigpen et al. demonstrated a similar design, but ICG was directly injected retrogradely into each ureteral catheter and the catheters were closed with caps to maximize ICG retention [10]. In our case, optimal visualization with Firefly technology was achieved by the combination of ureter stents and clamping of the bladder catheter after instillation of ICG. Compared with our study, the case report by Guan et al. showed some differences. Dissection of the serous and muscular layers of the bladder was performed using cystoscopic guidance with Firefly technology [9]. Another study described a robot-assisted transvaginal NOTES hysterectomy with resection of deeply infiltrating endometriosis. In their study, preoperatively 5 cc of ICG was injected after insertion of the ureteral stents [11].
Using the technique described in our case, ureterolysis of the distal ureters was not required during the lateral dissection of both the posterior bladder wall endometriosis and hysterectomy, given the clear enlarged vision of the fluorescent ureters with NIR imaging at the robot console (Supplementary Video). The risk of potential additional complications, including bleeding and nerve injury, during ureterolysis could be avoided. In addition, the operative time can be reduced by avoiding ureterolysis. The surgical approach is also easy reproducible.
Conflict of interest statement
Ph. Van Trappen is proctor for Intuitive Surgical, Inc. (Sunnyvale, CA, USA), and receives fees for proctoring, presentations and teaching. The authors have stated explicitly that otherwise there are no conflicts of interest in connection with this article.
Funding
None declared.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.



