-
PDF
- Split View
-
Views
-
Cite
Cite
Brandon M Larson, Ashish Francis, Daniel R McMahon, Eric Z Massanyi, Ananth Murthy, Simultaneous second-stage Fowler–Stephens Orchiopexy and microvascular testicular autotransplantation in a patient with prune belly syndrome with cryptorchidism, Journal of Surgical Case Reports, Volume 2023, Issue 10, October 2023, rjad588, https://doi.org/10.1093/jscr/rjad588
Close - Share Icon Share
Abstract
We present a case of simultaneous second-stage Fowler–Stephens Orchiopexy (FSO) with microvascular testicular autotransplantation for cryptorchidism and in a patient with prune belly syndrome. At 5 months old, the patient underwent laparoscopic bilateral first-stage FSO with the right testicle located 1 cm from the liver and the left slightly more caudal. An ultrasound on postoperative Day 72 following second-stage FSO and microvascular autotransplantation showed patent testicular vasculature. Our experience shows that this combination technique is safe and effective to supercharge the testicle and augment collateral vessels if clinical suspicion for monotherapy failure is high.
Introduction
Prune belly syndrome (PBS) is a rare disorder occurring in ~3.7 per 100 000 live male births. PBS is characterized by a triad of anomalies of abdominal wall musculature deficiency, bilateral cryptorchidism and urinary tract malformations [1]. The two-stage Fowler–Stephens Orchiopexy (FSO) is a surgical treatment of cryptorchidism, and the most common treatment if the teste does not have adequate length for tension-free scrotal placement [2]. The procedure mobilizes intra-abdominal testicles to the scrotal position, while preserving perfusion through augmented collateral circulation after gonadal vessel ligation [1]. An additional, uncommon treatment of cryptorchidism is microvascular autotransplantation. Traditionally, a monotherapy approach achieves reasonable success (78% overall) if the testes sit in a relatively caudal position and appear to have adequate collaterals [3].
Case report
A 2-year-old male with PBS presented after bilateral laparoscopic first-stage FSO at 5 months of age, during which the right testicle was located 1 cm inferior to the liver. This cephalad location raised concern the testicle would not survive on collateralized vessels alone following a second-stage FSO.
The epigastric vessels and testes were identified in their expected intra-abdominal location via laparoscopy prior to converting to laparotomy. The rectus muscles and deep epigastric vessels were delineated, and two lateral incisions were made through fascia (Fig. 1). Testicular vessels were ligated, and the testicles were transposed into the scrotum (Figs 2 and 3).
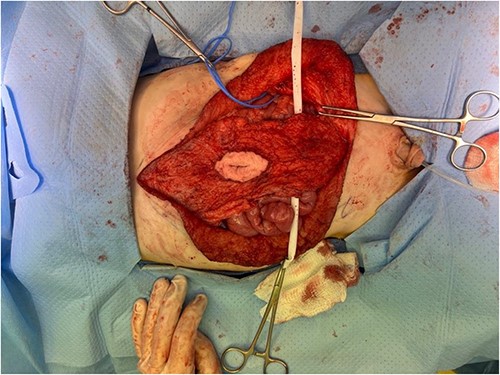
Surgical dissection of rectus muscles. A blue vessel loop encircles the right spermatic vessels.
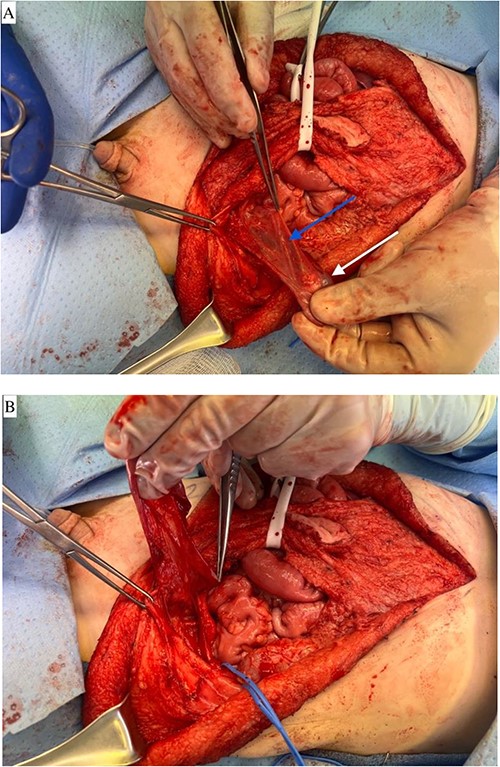
(A) Left testicle mobilized on the collateral vessels adjacent to the vas deferens. Blue arrow notes vas deferens and white arrow notes left testicle. (B) Left testicle mobilized on the collateral vessels adjacent to the vas deferens. Demonstrates a medial reflection of the mobilization depicted in A.
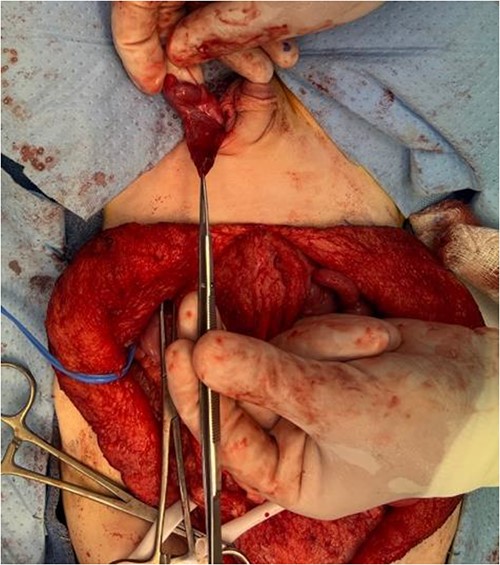
The left testicle was carefully brought down into the subdartos pouch and secured in place in the left hemiscrotum.
A myofascial flap was raised along the deep inferior epigastric vessels to the external iliac artery (Fig. 4A–C). The clamped edges of the testicular and deep inferior epigastric vessels were delivered through an inguinal incision to the scrotum. 10-0 interrupted nylon sutures were used for microvascular anastomosis of the right deep inferior epigastric and testicular arteries, followed by the right deep inferior epigastric vena comitans and testicular vein (Fig. 5).
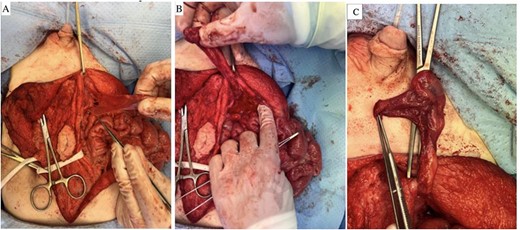
(A–C) Myofascial flap raised along the deep inferior epigastric vessels down to the level of the external iliac artery.
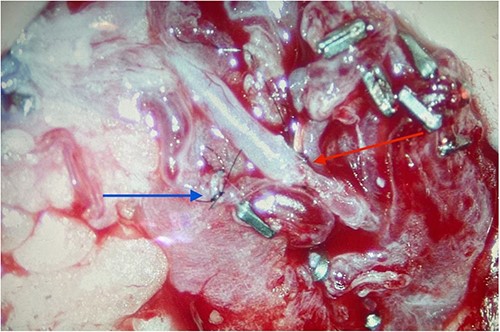
Completed microvascular anastomosis of deep inferior epigastric and testicular arteries using 10-0 interrupted nylon sutures (red arrow). Adjacent (blue arrow) is the completed anastomosis of the deep inferior epigastric vena comitans and testicular vein.
The postoperative course was unremarkable, and he was discharged home on postoperative Day 5. On postoperative Day 72, a duplex ultrasound demonstrated right testis size 1.5 × 0.8 × 0.8 cm (volume 0.5 mL) with position in the scrotal sac. Arterial and venous waveforms were noted on spectral Doppler imaging and color flow was comparable to the left side. Left testis size was 1.9 × 0.8 × 1.0 cm (volume 0.8 mL) with position in the scrotal sac.
Discussion
PBS is an uncommon condition first described by Frolich in 1839, and management of cryptorchidism has focused on either microvascular autotransplantation, or orchiopexy [4]. Fowler and Stephens [5] first described a single-stage testicular fixation after testicular vessel transection; however, the authors later transitioned to a two-stage open approach. Yu et al. describe an early adaption of laparoscopy to conduct the two-stage FSO with clip ligation of the spermatic vessels in a patient with PBS. At one and a half years old, the patient underwent the second stage, and both testicles were noted to be growing in intrascrotal position at 1-year follow-up [6].
Regardless of technique, a common goal is prevention of testicular atrophy. A meta-analysis of high-level intra-abdominal cryptorchidism compared outcomes of FSO as single-stage or two-stage, and open or laparoscopic. Overall success rates associated with single-stage and two-stage FSO were 85% and 87%, respectively. Rates of testicular atrophy were about 10% for both 1-stage and 2-stage FSO [7].
Early use of the operating microscope to successfully autotransplant an intra-abdominal teste to the scrotum in PBS was described by Silber and Kelly [8]. The spermatic vessels were ligated and anastomosed to “groin vessels” using 10-0 nylon suture. The authors conclude a microsurgical approach is a preferred alternative to a previously proposed technique conducted in dogs by Hodges et al., where the origin of the spermatic artery was transplanted with an aortic cuff to a lower position on the abdominal aorta [8, 9]. A more recent case series by Bukowski similarly describes microvascular anastomosis with 10-0 nylon sutures, and notes a 95% success rate, however, does not comment on criteria/objective evidence defining success [10].
Finally, Boddy et al. conducted a direct, retrospective comparison of the efficacy of FSO versus microvascular autotransplantation at a mean follow-up of 3 years. The authors showed 82% of testes after two-stage FSO were intrascrotal and viable, and 88% of microvascular transfers were successful [3].
The existing literature suggests an 80%–90% success rate for both two-stage FSO and microvascular testicular autotransplantation. Both techniques are supported as viable options for treating cryptorchidism in patients with PBS; however, prior to this case report there is no current literature describing both techniques performed simultaneously in a single operation. It is reasonable to suspect patients with very high intra-abdominal testes, or inadequate collateral vasculature may require both techniques. This case report describes such a patient, and demonstrates simultaneous FSO and microvascular autotransplantation is safe and efficacious in bilateral cryptorchidism. While postoperative Day 72 demonstrated patent arterial and venous flow of the microvascular anastomoses, further interval Doppler studies are required to determine long-term patency and absence of testicular atrophy. Future studies are required to definitively outline characteristics that might predispose patients to fall into the 10%–20% of patients that fail treatment with either FSO, or microvascular autotransplantation alone.
Conclusion
PBS with either high intra-abdominal testes or concern for poor collateral vessel development may be at risk of failing traditional techniques of FSO or microvascular autotransplantation alone. Our experience shows that simultaneous use of the two techniques is safe and effective. We propose this combination approach for patients at high risk of monotherapy failure, especially in cases of bilateral cryptorchidism.
Conflict of interest statement
None declared.
Funding
The authors have no relevant financial disclosures or conflicts of interest to report.



