-
PDF
- Split View
-
Views
-
Cite
Cite
Rasha Almnashef, Ruba Menchaf, Fatima A Idres, Ghina Aljammal, Marah Mansour, Mohamad Ali Farho, Zain A Ibrahim, Haitham Abbassi, Follicular thyroid carcinoma within a struma ovarii: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 10, October 2023, rjad584, https://doi.org/10.1093/jscr/rjad584
Close - Share Icon Share
Abstract
Struma ovarii comprises 1% of all ovarian tumors and 3% of ovarian teratomas. It occurs in older females. Struma ovarii is often asymptomatic, unilateral, and accidentally detected through abdominal ultrasound or computed tomography. It presents with palpable abdominal pain or irregular menstrual cycles. Generally, it is treated with surgical resection, even though the best procedure in these cases remains under discussion. In this study, we present a case of a 28-year-old female with severe pain in the right iliac fossa. Physical examination and radiological images showed a large mass. A bilateral salpingo-oophorectomy with omentectomy, a total mass resection, and an abdominal hysterectomy were performed. A biopsy confirmed the diagnosis of a follicular thyroid tumor. The management decision is based on clinical and pathological data. This is particularly challenging due to its rarity and the insufficient guidelines regarding the management of this type of cancer.
Introduction
Struma ovarii (SO) is a rare type of ovarian tumor, comprising 1% of all ovarian tumors and 3% of ovarian teratomas [1]. It represents a teratoma with over 50% mature thyroid tissue, but it occasionally appears with serous or mucous adenocytes [2, 3]. Although the vast majority of SOs are benign tumors, the possibility of malignancy can be as low as 5% [1]. Papillary and follicular thyroid carcinomas are the most common types. It is uncommon to observe other forms of thyroid cancer [1]. SO is often unilateral and more frequent among women between the ages of 40s and 60s [1]. Typically, SO is asymptomatic and is accidentally detected through abdominal ultrasound (US) or computed tomography (CT) [4]. SO symptoms are similar to those of other ovarian tumors, showing nonspecific characteristics such as palpable abdominal mass, abdominal pain, or irregular menstrual cycles [4]. Due to its rarity and limited research, there are still no established diagnostic or treatment guidelines. In general, benign unilateral tumors are treated with surgical resection [5]. In our case, we present a 29-year-old female patient with a follicular tumor at the expense of the ovarian stroma after an abdominal hysterectomy and bilateral salpingo-oophorectomy with omentectomy.
Case presentation
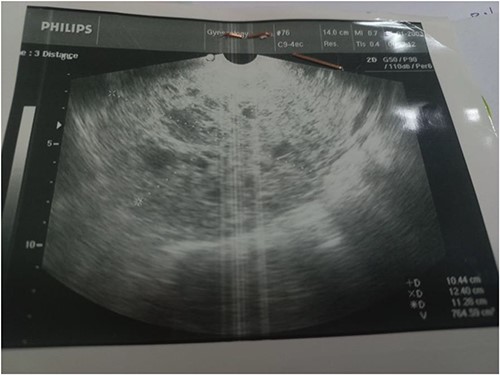
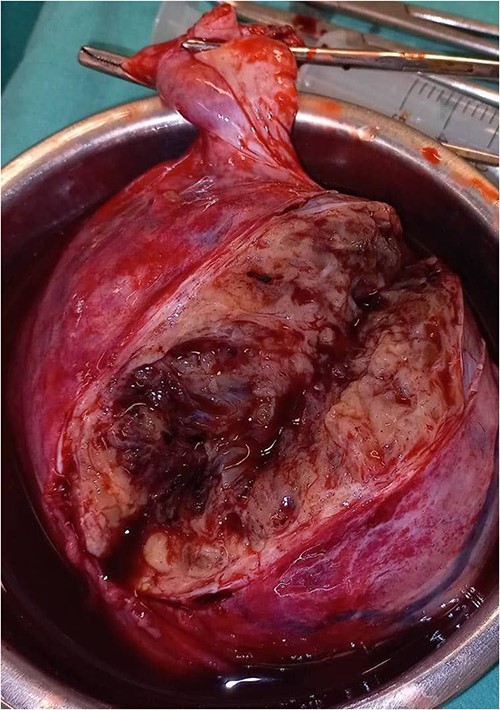
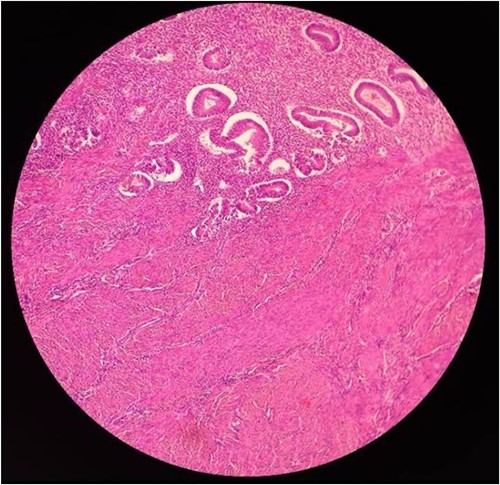
A 28-year-old female patient (G4B0) was admitted to the Department of Obstetrics and Gynecology with a history of severe pain in the right iliac fossa for several months, which had worsened for the last month. Physical examination revealed a large, palpable mass in the hypogastric region and the right lower abdominal quadrant. Vital signs, complete blood count, glucose, blood urea nitrogen, creatinine, alanine aminotransferase , aspartate aminotransferase, prothrombin time, activated partial thromboplastin time, and alpha-fetoprotein, were within normal limits. However, the CA-125 level was elevated at 52.8 U/mL. The vaginal US showed an unclear mass on the right side of the uterus with a similar appearance to a hydatidiform mole (Fig. 1). A pelvic CT scan with contrast showed a heterogeneous mass of 14 × 10 cm on the right side, containing several cystic vesicles lying in front of the uterus and displacing it posteriorly. There was no free fluid in the abdomen or pelvis. The patient’s condition deteriorated after 3 days, and she developed abdominal distension and mild tenderness. Surgery was performed, during which the entire mass was resected from the right ovary (Fig. 2). Histopathological examination revealed a follicular thyroid tumor originating from the right ovary (Fig. 3). Postoperative vital signs were within normal limits. The postoperative blood analyses were Hb = 9.7 g/dL, hematocrit (HCT): 28.5%, WBC: 10.4 × 10^9/L, neutrophils: 88%, and PLT: 182 × 10^9/L. Two units of blood were transfused, and the Hb level increased to 10.2 g/dL. Thyroid function tests, including thyroid-stimulating hormone and free thyroxine (FT4), were within normal limits. The patient was discharged in good condition after 5 days.
Discussion
Mature cystic teratomas represent ~20% of all ovarian tumors. Of these, ~15% compose thyroid tissue. SO is usually benign, is a monodermal variant of ovarian teratoma, contains mainly thyroid tissue (>50%), and accounts for 2.5 –5% of ovarian teratomas [6–9]. The papillary type (70%) is more common than the follicular type (30%) [9]. In this case, histology revealed a benign follicular struma (Fig. 3). SO generally occurs in females in the fourth to sixth decades of life [9], presenting predominantly as a unilateral adnexal mass, commonly involving the left ovary [6, 7]. Clinically and biochemically, euthyroidism is predominant in over 92% of patients with SO [7], while <8% of SO cases are affected by hyperthyroidism. These cases are usually mild and subclinical [10]. However, the preoperative diagnosis of SO was malignant. SO may be indicated through thyroglobulin titration or from scanning of hyperthyroidism patients [6]. Our patient had normal thyroid tests and an adnexal right mass. Matsuda et al. [10] reported on a 48-year-old female with symptomatic hyperthyroidism and an ovarian mass. Dunzendorfer et al. [11] presented only five SO cases and one with co-existing hyperthyroidism. Koehler et al. [8] reported on a 61-year-old female with Hashimoto’s thyroiditis on levothyroxine replacement therapy for years with a transition to clinical and biochemical hyperthyroidism despite antithyroid medication. A new diagnosis of urothelial carcinoma, and a adnexal mass. Asymptomatic cases account for 40% and are casually detected through routine US. While symptomatic patients present with palpable abdominal masses, abdominal pain, vaginal bleeding, tachycardia, and ascites [8]. Our patient complained of pain in the right iliac region with no response to analgesics. The US remains the first-choice imaging technique to evaluate ovarian disease [12]. The US remains the superior diagnostic tool for evaluating adnexal abnormalities [13]. Sonographic malignancy findings include irregular solids and ascites. At least four papillary structures, an irregular multilocular solid tumor with the largest diameter over 10 cm, and strong color flow [14]. Benign features are unilocular cysts of any size, solid components either not present or <7 mm in diameter, acoustic shadowing, smooth multilocular tumors with the largest diameter <10 cm, and no blood flow [14]. Unlike other neoplasms, adnexal masses do not require biopsies; therefore, imaging findings are crucial for diagnosis and management [15].
Conclusion
This case aims to highlight the complexity of this rare condition and the importance of early diagnosis and management. Customized medical decisions require considering both clinical and pathological findings. More research is needed to develop effective strategies for managing SO therapeutically.
Conflict of interest statement
None declared.
Funding
No funding was required.
Data availability
All data (of the patient) generated during this study are included in this published article and its supplementary information files.
Consent for publication
Written informed consent was obtained from the patient for publishing this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Guarantor
Marah Mansour is the guarantor of this work.
References
- abdominal pain
- computed tomography
- teratoma
- biopsy
- cancer
- physical examination
- follicular thyroid carcinoma
- ilium
- ovarian neoplasms
- pain
- struma ovarii
- thyroid neoplasms
- diagnosis
- thyroid
- hysterectomy, abdominal
- salpingo-oophorectomy
- omentectomy
- menstrual cycle, irregular
- abdominal ultrasonography
- excision



