-
PDF
- Split View
-
Views
-
Cite
Cite
Baraa Amir, Amaar Amir, Salwa Sheikh, High-grade appendiceal mucinous neoplasm presenting as renal colic; a case report and review of literature, Journal of Surgical Case Reports, Volume 2023, Issue 10, October 2023, rjad567, https://doi.org/10.1093/jscr/rjad567
Close - Share Icon Share
Abstract
High-grade appendiceal mucinous neoplasms (HAMN) are rare mucinous appendiceal tumors that are mostly incidentally discovered and histologically show marked cytoplasmic atypia. We report a 62-year-old female patient who was diagnosed with acute right flank pain mimicking renal colic. Abdominal and pelvis CT scans showed no calculi or hydronephrosis. Incidentally, a markedly distended retrocecal appendix was identified and an appendectomy was performed. Histopathology revealed an HAMN of size 5.8 cm. HAMN are one of the rare and somewhat recently described epithelial appendiceal tumors. The latest 2019 classification is based on histologic appearance and on the consensus for classification and pathologic reporting of Pseudomyxoma peritonei and associated appendiceal neoplasia (Peritoneal Surface Pathology Group International, modified Delphi process). Histologically, appendiceal mucinous lesions are classified as nonneoplastic/mucocele and neoplastic lesions with separate subcategorization. Despite its rarity, it is crucial for clinicians to be aware of HAMN to distinguish it from other clinical differentials.
Introduction
High-grade appendiceal mucinous neoplasms (HAMN) represent appendiceal tumors that are mucinous in nature and display a high degree of cytoplasmic atypia. Appendiceal tumors are rare and have an adjusted incidence of 0.12 cases for 1 000 000 person per year. They are usually incidentally discovered via radiology and are then classified histologically. HAMN are differentiated from low-grade appendiceal mucinous neoplasms (LAMN) through histology as radiological findings between the two types are indistinguishable. Herein we report a case of a female patient who presented with right flank pain mimicking renal colic. An abdominal and pelvic computed tomograohy (CT) ruled out renal calculi and hydronephrosis but a mucinous appendiceal mass was incidentally found that upon surgical resection and histopathologic examination was diagnosed to be HAMN.
Case presentation
A 62 -year-old Saudi female was referred to the emergency department with a history of acute right flank pain. The patient denied history of nausea, vomiting and fever. Physical exam showed tenderness on palpation on the right flank, but was otherwise unremarkable. Vital signs were stable. The patient’s past medical and surgical history were remarkable for hypertension, osteoporosis, tinea pedis, cholecystectomy and right parathyroidectomy for parathyroid adenoma. The clinical impression at that point was that her flank pain was indicative of renal colic.
Abdominal and pelvis unenhanced CT scan was performed, which showed no signs of renal or ureteric calculi. No hydronephrosis was present. A 2.4 cm simple cyst was detected in the right kidney. No other significant renal abnormalities were present. Incidentally, a distended retrocecal appendix was identified suggestive of a mucocele (Fig. 1). Accordingly, underlying neoplastic process needed to be excluded. No free gas or fluid was found in the peritoneal cavity and other intraabdominal organs and lymph nodes were unremarkable.
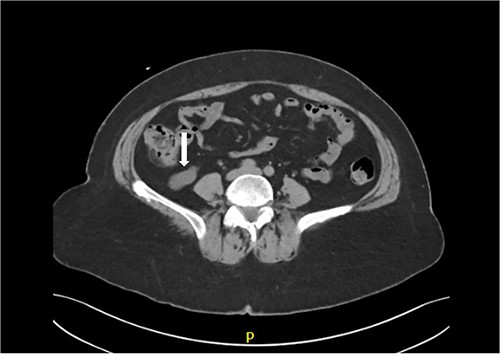
CT scan shows markedly dilated appendix consistent with mucocele.
The patient was recommended to undergo appendectomy and underwent elective laparoscopic appendectomy. Grossly, the appendix was intact with a tan and smooth serosal surface. The appendix measured 7.0 cm in length and varied in diameter ranging from 0.7 cm at the proximal margin to 2.6 cm distally. Close to the distal margin, the appendix was markedly dilated measuring 5.8 cm in diameter. The postoperative course of the patient was unremarkable. Histopathological examination of the appendix revealed the 5.8 cm dilated segment to represent a dilated appendix lined by dysplastic appendiceal epithelium producing abundant mucin and exhibiting expansile growth with pushing borders. Focal areas showed cribriform growth of the lining epithelium, nuclear stratification reaching the surface and high-grade nuclear features with enlarged atypical pleomorphic hyperchromatic nuclei with prominent mitotic activity. There was extension and involvement of tumor into the submucosa. There were no attached lymph nodes. Tumor pathologic stage classification was determined to be pT1, N0, M0 as subsequent assessment showed no other lesions (Fig. 2). The patient tolerated the procedure well and complained of mild postoperative pain. The patient was later discharged on the same day of the procedure in stable condition with instructions to follow up in surgery clinic.
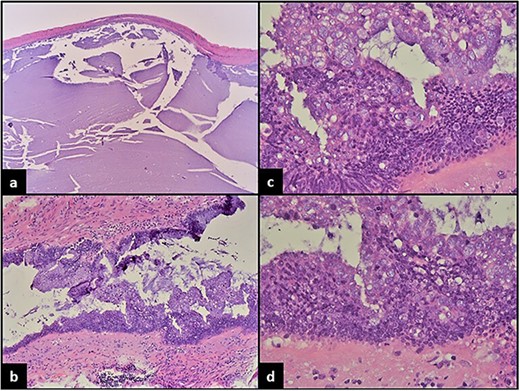
(a) Dilated appendix filled with mucinous material and mostly devoid of epithelial lining. (b) Focal areas show epithelial proliferation and piling up of cells forming vague papillary structures. (c) Marked convoluted architecture with focal cribriforming, epithelial proliferation and marked cytologic atypia. (d) High-grade dysplastic changes with nuclear stratification to the surface of the epithelium, large hyperchromatic pleomorphic nuclei and prominent mitosis.
Discussion
Primary malignant neoplasms of appendix are rare and show an age adjusted incidence of 0.12 cases for 1 000 000 person/year. Most of these neoplasms are incidentally discovered by radiology and or identified in approximately 1% of all appendectomy specimens. They are generally classified into nonneoplastic (mucocele) and neoplastic lesions with the latter consisting of three major histologic subtypes including mucinous neoplasms, nonmucinous neoplasms (colonic type), and goblet cell carcinoid tumor and the associated adenocarcinoma with predominant signet ring cell morphology [1]. The classification of appendiceal mucinous neoplasms has been controversial for many years with evolving nomenclature and considerable controversy. The latest individual 2019 classification is based on histologic appearance and not molecular characteristics and is based on the consensus for classification and pathologic reporting of Pseudomyxoma peritonei and associated appendiceal neoplasia (the results of the Peritoneal Surface Pathology Group International, modified Delphi process [2]. There have been in the past many classifications and staging schemes for primary appendiceal mucinous tumors and their associated P. peritonei using histologic and clinical characteristics with controversial terminologies. The challenge remained regarding diagnostic designation with appropriate staging and treatment of these lesions. Appendiceal mucinous lesions are classified pathologically as nonneoplastic (mucocele that is considered as clinical term) and neoplastic epithelial lesions with separate categorization into various types. The current pathologic classification scheme for neoplastic lesions takes into account both the histopathologic features of the epithelium and the pattern of mural involvement [3].
Appendiceal mucinous neoplasm is the most common epithelial tumor of appendix that accounts for approximately 33% of all appendiceal neoplasm. The simple mucocele also termed as retention cyst is believed to be cystic dilatation of the appendix secondary to obstruction with normal epithelial lining lacking any dysplastic features and, therefore, is not considered as a neoplastic process.
The neoplastic appendiceal mucinous lesions include serrated lesions and hyperplastic polyps, LAMN, HAMN and mucinous adenocarcinoma [4, 5]. Both LAMN and HAMN, by definition, lack infiltrative growth pattern with the former exhibiting low-grade epithelial dysplastic features and the latter showing high-grade cytologic atypia.
LAMN, by consensus, is described as a tumor with dysplastic appendiceal epithelium producing abundant mucin and exhibiting expansile growth with pushing borders, which may or may not cause loss of muscular component wall and mural fibrosis [6]. It always lacks clear infiltrative growth, and although mucin dissection through the appendiceal wall may be seen, the mucin pools are acellular. The lining epithelial cells show low-grade dysplastic changes with mildly enlarged hyperchromatic nuclei, and minimal mitotic activity.
HAMN are indistinguishable from LAMN with both lacking an infiltrative growth pattern; however, HAMN show high-grade dysplastic features including cribriform growth, nuclear stratification to the surface of epithelium, high-grade nuclear features exhibiting enlarged atypical pleomorphic hyperchromatic nuclei and abundant mitotic activity [6]. The neoplastic epithelium lining both the above lesions are positive for CK20, CDX2, and SATB2 and negative for PAX8. They frequently Harbor K-ras mutations (Kirsten rat sarcoma virus) and show GNAS alterations (Guanine Nucleotide binding protein, Alpha Stimulating activity polypeptide) in 50% of cases. The loss of chromosome 5q has also been reported. In addition, HAMN may show TP53 and ATM mutations (Ataxia-Telangiectasia Mutated) [7–9].
HAMN is a recently created pathologic category as previous WHO classification categorized mucinous appendiceal lesions into adenomas and LAMN referring to lesions confined to the appendix, and mucinous low- and high-grade carcinomas for those exhibiting spread to the peritoneum [1]. HAMN is believed to represent an aggressive form of mucinous neoplasm that is more likely to be associated with high-grade disease in the peritoneum. There are limited data on HAMN’s clinical prognostic behavior and therefore it is staged as adenocarcinoma and is still considered a highly controversial area [10]. However, to date, the current recommended management is the same for both entities which is standard appendectomy. Patients with LAMN or HAMN that are confined to appendix and are not ruptured are completely excised and do not require hemicolectomy.
Both LAMN and HAMN are more common in women and typically present in the sixth decade. Most patients with tumor confined to appendix present with symptoms similar to acute appendicitis. Tumors with disseminated disease often present with abdominal pain and mass, ovarian mass and/or with pseudomyxoma peritonei. Radiologically, an appendix with more than 1.5 cm diameter, wall thickening and/or with soft tissue mass raise the possibility of a mucinous neoplasm.
The 8th edition American Joint Committee on Cancer staging manual provides guidelines for staging both LAMN and HAMN with consideration of HAMN representing adenocarcinomas for staging purposes The same is also reflected in College of American pathology protocol for reporting. LAMN confined to the appendix without invasion of muscularis propria is classified as Tis (LAMN), and T1 and T2 stages are not used for LAMN; however, lesions that demonstrate involvement of subserosa or serosa by acellular mucin are classified as T3 or T4a, respectively; mucin involvement of distant peritoneal sites is classified as M1. HAMN, on the other hand, is staged as invasive adenocarcinoma (T1–T4) because of their higher risk of recurrence. However, this area is controversial and remains unclear with more data and studies needed for further understanding of this tumor [3, 10].
The old WHO classification divided mucinous appendiceal lesions into those confined to the appendix (adenoma and LAMN) and lesions with spread to the peritoneum as carcinomas. The recently introduced term ‘appendiceal mucinous lesions’ is considered simple and reasonably suitable to distinguish between lesions with and without potential of malignancy and dissemination. Regardless of the controversy of different prognostic behavior of LAMN and HAMN, the recommended treatment for both these localized lesions without evidence of rupture remains standard appendectomy. Ruptured tumors and adenocarcinomas require advanced radical surgical management.
As a rare and relatively recent entity, there is still much to know about HAMN since the research conducted regarding this topic is few and far between. Despite its rarity, it is crucial to distinguish it from other clinical and histologic mimickers and to identify it correctly by histopathologic examination. Herein we report a case of a 62-year-old female who was diagnosed with right flank pain mimicking renal colic. Radiologically, a mucinous appendiceal lesion was discovered incidentally that upon resection was identified to be HAMN. As a relatively new entity, there is still much to know about HAMN and awareness of clinicians and pathologists of this entity is of utmost significance to diagnose it correctly. In addition, we recommend further larger studies to better understand the complexity and behavior of mucinous neoplasms in order to correlate with the long-term prognosis of each category in particular HAMN.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article. Submitting authors are responsible for coauthors declaring their interests.
Funding
No funding or grant support.
Data availability
The data used to support the findings of this study are included within the article.



