-
PDF
- Split View
-
Views
-
Cite
Cite
Leva Gorji, Andrew Archer, Dermatofibrosarcoma protuberans: a case report of an abdominal wall mass and review of the literature, Journal of Surgical Case Reports, Volume 2023, Issue 10, October 2023, rjad542, https://doi.org/10.1093/jscr/rjad542
Close - Share Icon Share
Abstract
Dermatofibrosarcoma protuberans (DFSP) is a rare, slow-growing malignancy that often presents with an ambiguous clinical presentation due to its nonspecific findings. We present the case of a 41-year-old male who presented with a slow-growing mass on his abdomen that became protuberant and firm, prompting his desire to pursue excision. Upon return of histologic examination, the specimen was noted to be DFSP. DFSP is a rare cutaneous neoplasm that originates from the dermis and invades the underlying tissue creating a classic protuberant appearance. In some cases, chemotherapy and radiation may be indicated based on margins and locations. The sarcoma is notoriously complicated with early recurrence, making the disease process difficult to control. Surgeons should be familiar with this malignancy due to the indication of additional nonsurgical treatments and the necessity for long-term follow-up for surveillance of recurrence.
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare soft tissue tumor that accounts for nearly 1% of all malignancies [1]. The cutaneous is typically confined to the skin and subcutaneous tissue; however, it may also invade the muscle and fascia. The slow-growing neoplasm often manifests in the trunk and proximal extremities between the third and fourth decades of life. While the exact cause of tumorigenesis is not entirely known, the properties of the tumor result in the upregulation of platelet-derived growth factor B (PDGFB) expression and self-propagation of DFSP cells [2].
Case report
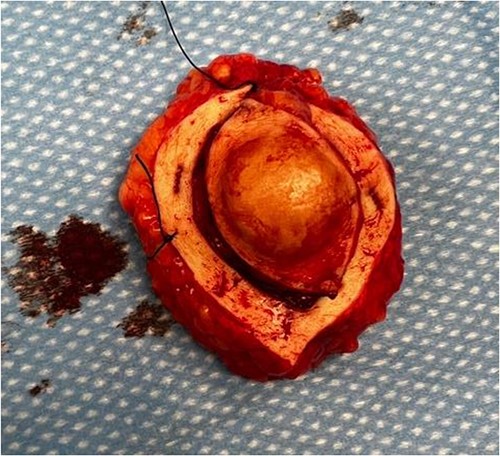
We present the case of a 41-year-old white male with a history of IV drug abuse who presented with a left-sided abdominal mass, which he noticed one year prior to presentation. The patient did attempt to lance the mass due to concerns that it may be an enlarging abscess; however, the mass demonstrated minimal blood output as a result of the drainage attempt. On physical exam, the mass was noted to be a firm, tender, circumferential, and non-erythematous 5 × 3 cm protuberant mass. The patient had no relevant past surgical or family history. Intraoperatively, an elliptical incision was made surrounding the protuberant mass, and ultimately the incision was extended in all directions as the capsule of the mass extended laterally beyond the exophytic portion that protruded through the abdominal wall (Fig. 1). The mass and its capsule were removed in entirety peripherally and to its deep margin overlying the anterior rectus sheath. On microscopic evaluation, the spindle cells demonstrated positive staining for CD34, but negative staining for desmin, CD117, and S100.
Discussion
It is postulated that the tumor occurs due to the translocation of (17;22)(q22;q13), which results in a fusion of alpha-chain type 1 collagen (COL1A1) and PFGFB and, in turn, results in constitutive expression of PDGFB [3]. Notably, there is often heterogeneity in PDGFB expression throughout the tumor tissue itself [3]. Nakamura et al. further investigated the proposal that DFSP is a PDFGB-dependent tumor with a 30-patient study. A total of 28 of 30 (93%) patients demonstrated the formation of a fusion gene between COL1A1 and PDGFB, and 29 of 30 (97%) expressed CD34 and nestin [4].
Various imaging modalities may be utilized to characterize these lesions, including ultrasound and MRI. However, on imaging, DFSPs are difficult to differentiate from other soft-tissue neoplasms. Therefore, tissue sampling and histology are imperative for definitive diagnosis. Tumors typically stain positive for vimentin, CD34, apolipoprotein D, nestin, and negative for desmin, s100 protein, Factor XIIIa, stromeylsin III, CD163, and keratins [5–10]. On microscopic evaluation, DFSP tumor cells are typically arranged in a storiform fashion parallel to the epidermal surface with limited cytoplasm; there is a characteristic honeycombing appearance. However, eight other histologic subtypes of DFSP also exist [10].
Wide local excision with negative margins is recommended when feasible; however, Mohs surgery with negative margins has been described for cosmetic preservation, as well [11]. Nonetheless, the neoplasm is plagued with recurrence, with nearly half occurring in the first year following resection [12]. As a result, patients should undergo surveillance with a physical exam every 6–12 months to monitor for recurrence [11]. In the setting of metastatic, recurrent, or unresectable disease, chemotherapy with imatinib is indicated. Although the duration of treatment is not well-established, the oral tyrosine kinase inhibitor restricts proliferation and encourages apoptosis. Imatinib is particularly beneficial in patients who demonstrate t(17,22), which may be detected using fluorescent in situ hybridization or reverse transcription-polymerase chain reaction (Table 1) [13]. Radiation therapy (RT) may also be utilized in the setting of non-Mohs excisions with <1 cm margins. 50–60 Gy can be utilized for positive margins; ideally, the radiation field should extend 3–5 cm beyond the surgical margins when feasible [11]. Furthermore, RT may also be a suitable route of care when surgical treatment options have been exhausted in the setting of metastatic or recurrent disease without previous radiation administration [11].
| Author . | Study period . | N . | Study characteristics . | Outcomes . |
|---|---|---|---|---|
| Chemotherapy | ||||
| Han et al. [14] | 2004–2007 | 4 | 800 mg/d of imatinib (n = 2), 600 mg/d (n = 1), 400 mg/d (n = 1). | Neoadjuvant imatinib resulted in tumor size reduction of 36.9%, and resulted in 100% local control following Mohs surgery at a maximum f/u of 4 yrs. |
| McArthur et al. [15] | 10 | 800 mg/d of imatinib in locally advanced (n = 8) and metastatic (n = 2) DFSP. | All 8 pts w/ locally advanced dsx had t(17;22) and showed CR to imatinib, w/ 4 showing complete CR. 1 pt w/ metastatic DFSP + t(17;22) had a PR but experienced DP after 7 mo; the other pt w/ metastatic dsx lacking t(17;22) had no CR. | |
| Rutkowski et al. [16] | 2004–2014 | 31 | 800 mg/d of imatinib in inoperable (n = 16) or metastatic (n = 15) DFSP w/ cytogenetically confirmed COL1A1-PDGFB fusion. | 5-year PFS was 58%, 5-year OS was 64%. FS-DFSP and presence of metastases was asscx w shorter PFS and OS. |
| Stacchiotti et al. [17] | 2007–2015 | 10 | 400 mg/d of imatinib, increased to 800 mg/d w/ resistance. All pts with metastatic relapse from DFSP/FS-DFSP. | Median PFS was 11 mos. 5 pts underwent surgery after imatinib, and all relapsed. |
| Ugurel et al. [18] | 2004–2006 | 14 | 600 mg/d of imatinib for at least 6 weeks; at 12 weeks, pts underwent surgery or continuation of imatinib at investigator discretion. Tx was stopped at any point due to DP or AEs. Median duration of 3.1 mos. | At 12 week evaluation, 7 pts showed PR, 5 pts with SD, and 2 pts with PD. TR was measured w/ CT, MRI, ultrasound, or photography. |
| Wang et al. [19] | 2009–2014 | 22 | 400 mg/d of imatinib, increased to 800 mg/d w/ resistance. Pts with inoperable (n = 10) or metastatic DFSP (n = 12). | Median PFS was 19 mos. 1- and 3-year OS was 95.5 and 77.3%, respectively. Of the 10 pts w/ inoperable dsx, 4 were able to undergo complete surgical resection. |
| Radiation | ||||
| Castle et al. [20] | 1972–2010 | 53 | 13% with preop RT (50–50.4 Gy), 87% with postop RT (60–66 Gy). | Local control at 5- and 10-years were 98 and 93%, OS 98 and 98%, DFS was 98 and 93%, respectively. |
| Du et al. [21] | 2000–2016 | 184 | Surgery vs. Surgery + RT. | Age ≥ 50, margins <2 cm, and tumor size >5 cm asscx w worse DFS (P = 0.002, P = 0.030, P = 0.032, respectively). DFS in the surgery group vs. Surgery + RT was 56.2 vs. 88.1% (P = 0.044), respectively. Furthermore Ki-67 < 17% vs. ≥17% resulted in DFS of 87.8 vs. 35.8% (P = 0.002). DFS was measured at 5 years. |
| Wang et al. [22] | 1990–1999 | 74 | Surgery vs. Surgery + RT | 5-year RFS with surgery vs. surgery + RT was 58 vs. 90%, respectively. |
| Woo et al. [23] | 1999–2011 | 63 | Group I - marginal excision; group II – resection margins <3 cm; group III – resection margins >3 cm. | Recurrence was greater with group I than group II/III (35.7 vs. 0%, P < 0.001) at 65 mo f/u. Group III had higher rates of reconstruction than group II (82.7 vs. 52.4%, P = 0.011). Adjuvant RT was asscx w/ reduced recurrence in group I (0 vs. 60%, P = 0.016). |
| Author . | Study period . | N . | Study characteristics . | Outcomes . |
|---|---|---|---|---|
| Chemotherapy | ||||
| Han et al. [14] | 2004–2007 | 4 | 800 mg/d of imatinib (n = 2), 600 mg/d (n = 1), 400 mg/d (n = 1). | Neoadjuvant imatinib resulted in tumor size reduction of 36.9%, and resulted in 100% local control following Mohs surgery at a maximum f/u of 4 yrs. |
| McArthur et al. [15] | 10 | 800 mg/d of imatinib in locally advanced (n = 8) and metastatic (n = 2) DFSP. | All 8 pts w/ locally advanced dsx had t(17;22) and showed CR to imatinib, w/ 4 showing complete CR. 1 pt w/ metastatic DFSP + t(17;22) had a PR but experienced DP after 7 mo; the other pt w/ metastatic dsx lacking t(17;22) had no CR. | |
| Rutkowski et al. [16] | 2004–2014 | 31 | 800 mg/d of imatinib in inoperable (n = 16) or metastatic (n = 15) DFSP w/ cytogenetically confirmed COL1A1-PDGFB fusion. | 5-year PFS was 58%, 5-year OS was 64%. FS-DFSP and presence of metastases was asscx w shorter PFS and OS. |
| Stacchiotti et al. [17] | 2007–2015 | 10 | 400 mg/d of imatinib, increased to 800 mg/d w/ resistance. All pts with metastatic relapse from DFSP/FS-DFSP. | Median PFS was 11 mos. 5 pts underwent surgery after imatinib, and all relapsed. |
| Ugurel et al. [18] | 2004–2006 | 14 | 600 mg/d of imatinib for at least 6 weeks; at 12 weeks, pts underwent surgery or continuation of imatinib at investigator discretion. Tx was stopped at any point due to DP or AEs. Median duration of 3.1 mos. | At 12 week evaluation, 7 pts showed PR, 5 pts with SD, and 2 pts with PD. TR was measured w/ CT, MRI, ultrasound, or photography. |
| Wang et al. [19] | 2009–2014 | 22 | 400 mg/d of imatinib, increased to 800 mg/d w/ resistance. Pts with inoperable (n = 10) or metastatic DFSP (n = 12). | Median PFS was 19 mos. 1- and 3-year OS was 95.5 and 77.3%, respectively. Of the 10 pts w/ inoperable dsx, 4 were able to undergo complete surgical resection. |
| Radiation | ||||
| Castle et al. [20] | 1972–2010 | 53 | 13% with preop RT (50–50.4 Gy), 87% with postop RT (60–66 Gy). | Local control at 5- and 10-years were 98 and 93%, OS 98 and 98%, DFS was 98 and 93%, respectively. |
| Du et al. [21] | 2000–2016 | 184 | Surgery vs. Surgery + RT. | Age ≥ 50, margins <2 cm, and tumor size >5 cm asscx w worse DFS (P = 0.002, P = 0.030, P = 0.032, respectively). DFS in the surgery group vs. Surgery + RT was 56.2 vs. 88.1% (P = 0.044), respectively. Furthermore Ki-67 < 17% vs. ≥17% resulted in DFS of 87.8 vs. 35.8% (P = 0.002). DFS was measured at 5 years. |
| Wang et al. [22] | 1990–1999 | 74 | Surgery vs. Surgery + RT | 5-year RFS with surgery vs. surgery + RT was 58 vs. 90%, respectively. |
| Woo et al. [23] | 1999–2011 | 63 | Group I - marginal excision; group II – resection margins <3 cm; group III – resection margins >3 cm. | Recurrence was greater with group I than group II/III (35.7 vs. 0%, P < 0.001) at 65 mo f/u. Group III had higher rates of reconstruction than group II (82.7 vs. 52.4%, P = 0.011). Adjuvant RT was asscx w/ reduced recurrence in group I (0 vs. 60%, P = 0.016). |
Abbreviations: AEs, adverse events; asscx, associated; DFSP, dermatofibrosarcoma protuberans; DFS, disease-free survival; DP, disease progression; FS-DFSP, fibrosarcomatous transformation dermatofibrosarcoma protuberans; F/u, follow up; – mos, months; OS, overall survival; PR, partial response; PD, progressive disease; RT, radiation therapy; Tx, treatment; TR, tumor response.
| Author . | Study period . | N . | Study characteristics . | Outcomes . |
|---|---|---|---|---|
| Chemotherapy | ||||
| Han et al. [14] | 2004–2007 | 4 | 800 mg/d of imatinib (n = 2), 600 mg/d (n = 1), 400 mg/d (n = 1). | Neoadjuvant imatinib resulted in tumor size reduction of 36.9%, and resulted in 100% local control following Mohs surgery at a maximum f/u of 4 yrs. |
| McArthur et al. [15] | 10 | 800 mg/d of imatinib in locally advanced (n = 8) and metastatic (n = 2) DFSP. | All 8 pts w/ locally advanced dsx had t(17;22) and showed CR to imatinib, w/ 4 showing complete CR. 1 pt w/ metastatic DFSP + t(17;22) had a PR but experienced DP after 7 mo; the other pt w/ metastatic dsx lacking t(17;22) had no CR. | |
| Rutkowski et al. [16] | 2004–2014 | 31 | 800 mg/d of imatinib in inoperable (n = 16) or metastatic (n = 15) DFSP w/ cytogenetically confirmed COL1A1-PDGFB fusion. | 5-year PFS was 58%, 5-year OS was 64%. FS-DFSP and presence of metastases was asscx w shorter PFS and OS. |
| Stacchiotti et al. [17] | 2007–2015 | 10 | 400 mg/d of imatinib, increased to 800 mg/d w/ resistance. All pts with metastatic relapse from DFSP/FS-DFSP. | Median PFS was 11 mos. 5 pts underwent surgery after imatinib, and all relapsed. |
| Ugurel et al. [18] | 2004–2006 | 14 | 600 mg/d of imatinib for at least 6 weeks; at 12 weeks, pts underwent surgery or continuation of imatinib at investigator discretion. Tx was stopped at any point due to DP or AEs. Median duration of 3.1 mos. | At 12 week evaluation, 7 pts showed PR, 5 pts with SD, and 2 pts with PD. TR was measured w/ CT, MRI, ultrasound, or photography. |
| Wang et al. [19] | 2009–2014 | 22 | 400 mg/d of imatinib, increased to 800 mg/d w/ resistance. Pts with inoperable (n = 10) or metastatic DFSP (n = 12). | Median PFS was 19 mos. 1- and 3-year OS was 95.5 and 77.3%, respectively. Of the 10 pts w/ inoperable dsx, 4 were able to undergo complete surgical resection. |
| Radiation | ||||
| Castle et al. [20] | 1972–2010 | 53 | 13% with preop RT (50–50.4 Gy), 87% with postop RT (60–66 Gy). | Local control at 5- and 10-years were 98 and 93%, OS 98 and 98%, DFS was 98 and 93%, respectively. |
| Du et al. [21] | 2000–2016 | 184 | Surgery vs. Surgery + RT. | Age ≥ 50, margins <2 cm, and tumor size >5 cm asscx w worse DFS (P = 0.002, P = 0.030, P = 0.032, respectively). DFS in the surgery group vs. Surgery + RT was 56.2 vs. 88.1% (P = 0.044), respectively. Furthermore Ki-67 < 17% vs. ≥17% resulted in DFS of 87.8 vs. 35.8% (P = 0.002). DFS was measured at 5 years. |
| Wang et al. [22] | 1990–1999 | 74 | Surgery vs. Surgery + RT | 5-year RFS with surgery vs. surgery + RT was 58 vs. 90%, respectively. |
| Woo et al. [23] | 1999–2011 | 63 | Group I - marginal excision; group II – resection margins <3 cm; group III – resection margins >3 cm. | Recurrence was greater with group I than group II/III (35.7 vs. 0%, P < 0.001) at 65 mo f/u. Group III had higher rates of reconstruction than group II (82.7 vs. 52.4%, P = 0.011). Adjuvant RT was asscx w/ reduced recurrence in group I (0 vs. 60%, P = 0.016). |
| Author . | Study period . | N . | Study characteristics . | Outcomes . |
|---|---|---|---|---|
| Chemotherapy | ||||
| Han et al. [14] | 2004–2007 | 4 | 800 mg/d of imatinib (n = 2), 600 mg/d (n = 1), 400 mg/d (n = 1). | Neoadjuvant imatinib resulted in tumor size reduction of 36.9%, and resulted in 100% local control following Mohs surgery at a maximum f/u of 4 yrs. |
| McArthur et al. [15] | 10 | 800 mg/d of imatinib in locally advanced (n = 8) and metastatic (n = 2) DFSP. | All 8 pts w/ locally advanced dsx had t(17;22) and showed CR to imatinib, w/ 4 showing complete CR. 1 pt w/ metastatic DFSP + t(17;22) had a PR but experienced DP after 7 mo; the other pt w/ metastatic dsx lacking t(17;22) had no CR. | |
| Rutkowski et al. [16] | 2004–2014 | 31 | 800 mg/d of imatinib in inoperable (n = 16) or metastatic (n = 15) DFSP w/ cytogenetically confirmed COL1A1-PDGFB fusion. | 5-year PFS was 58%, 5-year OS was 64%. FS-DFSP and presence of metastases was asscx w shorter PFS and OS. |
| Stacchiotti et al. [17] | 2007–2015 | 10 | 400 mg/d of imatinib, increased to 800 mg/d w/ resistance. All pts with metastatic relapse from DFSP/FS-DFSP. | Median PFS was 11 mos. 5 pts underwent surgery after imatinib, and all relapsed. |
| Ugurel et al. [18] | 2004–2006 | 14 | 600 mg/d of imatinib for at least 6 weeks; at 12 weeks, pts underwent surgery or continuation of imatinib at investigator discretion. Tx was stopped at any point due to DP or AEs. Median duration of 3.1 mos. | At 12 week evaluation, 7 pts showed PR, 5 pts with SD, and 2 pts with PD. TR was measured w/ CT, MRI, ultrasound, or photography. |
| Wang et al. [19] | 2009–2014 | 22 | 400 mg/d of imatinib, increased to 800 mg/d w/ resistance. Pts with inoperable (n = 10) or metastatic DFSP (n = 12). | Median PFS was 19 mos. 1- and 3-year OS was 95.5 and 77.3%, respectively. Of the 10 pts w/ inoperable dsx, 4 were able to undergo complete surgical resection. |
| Radiation | ||||
| Castle et al. [20] | 1972–2010 | 53 | 13% with preop RT (50–50.4 Gy), 87% with postop RT (60–66 Gy). | Local control at 5- and 10-years were 98 and 93%, OS 98 and 98%, DFS was 98 and 93%, respectively. |
| Du et al. [21] | 2000–2016 | 184 | Surgery vs. Surgery + RT. | Age ≥ 50, margins <2 cm, and tumor size >5 cm asscx w worse DFS (P = 0.002, P = 0.030, P = 0.032, respectively). DFS in the surgery group vs. Surgery + RT was 56.2 vs. 88.1% (P = 0.044), respectively. Furthermore Ki-67 < 17% vs. ≥17% resulted in DFS of 87.8 vs. 35.8% (P = 0.002). DFS was measured at 5 years. |
| Wang et al. [22] | 1990–1999 | 74 | Surgery vs. Surgery + RT | 5-year RFS with surgery vs. surgery + RT was 58 vs. 90%, respectively. |
| Woo et al. [23] | 1999–2011 | 63 | Group I - marginal excision; group II – resection margins <3 cm; group III – resection margins >3 cm. | Recurrence was greater with group I than group II/III (35.7 vs. 0%, P < 0.001) at 65 mo f/u. Group III had higher rates of reconstruction than group II (82.7 vs. 52.4%, P = 0.011). Adjuvant RT was asscx w/ reduced recurrence in group I (0 vs. 60%, P = 0.016). |
Abbreviations: AEs, adverse events; asscx, associated; DFSP, dermatofibrosarcoma protuberans; DFS, disease-free survival; DP, disease progression; FS-DFSP, fibrosarcomatous transformation dermatofibrosarcoma protuberans; F/u, follow up; – mos, months; OS, overall survival; PR, partial response; PD, progressive disease; RT, radiation therapy; Tx, treatment; TR, tumor response.
Conclusion
In the event where preoperative tissue diagnosis is present, neoadjuvant chemotherapy may be beneficial in addressing the neoplasm. However, surgical excision remains with wide margins remains the standard for interventions currently. The neoplasm does demonstrate radiosensitivity, which may be used in conjunction with surgical excision. However, ultimately following treatment surveillance is imperative due to a high risk of recurrence.
Conflict of interest statement
None declared.
Funding
None declared.



