-
PDF
- Split View
-
Views
-
Cite
Cite
José Sergio Verboonen Sotelo, Jeffry Romero Manzano, Guillermo Vega Tostado, José Aldo Guzmán Barba, Isaac Esparza Estrada, José Oscar Orozco Álvarez Malo, Alejandro González Ojeda, One-anastomosis gastric bypass reversal due to severe malnutrition and acute hepatic failure: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 1, January 2023, rjad009, https://doi.org/10.1093/jscr/rjad009
Close - Share Icon Share
Abstract
The one-anastomosis gastric bypass (OAGB) is one of the most popular performed bariatric surgeries and has good long-term success for treating obesity and metabolic diseases. However, some patients can develop severe complications such as malnutrition and hepatic steatosis, which can be corrected with a reversal procedure, as seen in this case. A 20-year-old woman underwent OAGB surgery, which was converted to Roux-en-Y gastric bypass 4 months after the initial procedure due to malnutrition, both surgeries were performed at a hospital in southern Mexico. After the second surgery, she presented to our hospital with intolerance to oral feeding, vomiting and loss of 44 kg in 4 months. The patient was stabilized and scheduled for reversion surgery to normal anatomy 5 months later. She had good short-term nutritional outcomes and at the 1-year follow-up her total weight gain was 14 kg.
INTRODUCTION
Bariatrics is a branch of surgery that has grown significantly during the past decades because of its effectiveness in treating specific conditions such as obesity, diabetes and hypertension [1]. One-anastomosis gastric bypass (OAGB) is one of the bariatric surgeries that has been growing in popularity, along with the Roux-en-Y gastric bypass (RYGB). These restrictive and malabsorptive procedures lead to considerable decreases in comorbidities and cause massive weight loss [2]. Severe complications are uncommon, in some series lower than 0.5% [3]. While lengthening the biliopancreatic limb may facilitate weight loss, it also shortens the alimentary limb/common intestinal channel, which may exacerbate malabsorption and nutritional deficits [4].
Although the limited literature on the reversal procedures, they have been proposed as an effective treatment for patients with severe complications. However, these procedures should be performed by not only skilled surgeons but structured bariatric multidisciplinary teams and quality hospitals with ICU, interventional radiology and surgical endoscopy teams [5].
CASE REPORT
A 20-year-old woman underwent to a conversion from OAGB to RYGB due to severe malnutrition without improvement. Before OAGB, she weighed 97 kg, measured 150 cm and her body mass index (BMI) was 43.1, after the first surgery she had a loss of 19 kg. When she first came to our hospital, she weighed 34 kg, her BMI was 15.11 kg/m2, she had intolerance to oral feeding, vomiting and loss of 44 kg in 4 months. She had no history of chronic degenerative diseases or drug addiction (Fig. 1).
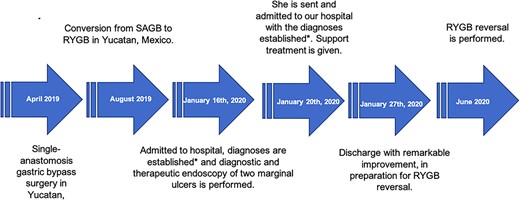
*Severe hepatic steatosis, severe protein–calorie malnutrition, anemia III, water–electrolyte imbalance and uncomplicated urinary tract infection.
The physical examination showed jaundice, decreased respiratory sounds, extremities with decreased sensitivity and edema. Laboratory tests showed a hemoglobin of 9.8 g/dL, hematocrit 30.5%, sodium 131 mmol/L, potassium 3.7 mmol/L, chlorine 95 mmol/L, total bilirubin 6.54 mg/dL, direct bilirubin 5.53 mg/dL, indirect bilirubin 1.01 mg/dL, aspartate transaminase (AST) 84 U/L, alanine transaminase 29 U/L, alkaline phosphatase 153 U/L, albumin 2.59 g/dL, gamma-glutamyl transferase 110 U/L, prothrombin time 20.6 s (control 13.6 s), partial thromboplastin time 38.4 s (control 29 s), INR 1.53. Viral serology and tumor markers and antibodies were negative.
Endoscopy identified two marginal ulcers measuring 3–4 cm located posterior to the gastrojejunal anastomosis. Ultrasound showed severe hepatic steatosis, ascites and biliary sludge. Because of anemia, two blood transfusions and anti-ammonia measures were administered.
She was diagnosed with severe hepatic steatosis, peripheral neuropathy, severe protein–calorie malnutrition and anemia III according to World Health Organization scale [6], hydroelectrolyte imbalance, ascites grade 1 and urinary infection. Given the severity of malnutrition and intolerance to oral administration, a parenteral diet of 10–15 kcal/day including protein of 1–1.5 g/kg was started. The patient was given vitamin K and diuretics. On Day 15, the patient was discharged to receive outpatient treatment in collaboration with gastrointestinal and nutrition specialists. She returned 5 months later for a reversal of the RYBG.
SURGICAL REPORT
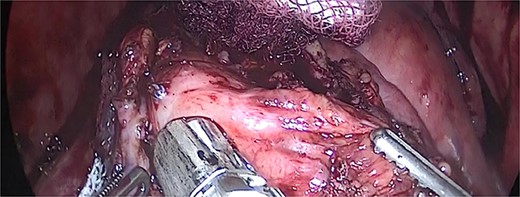
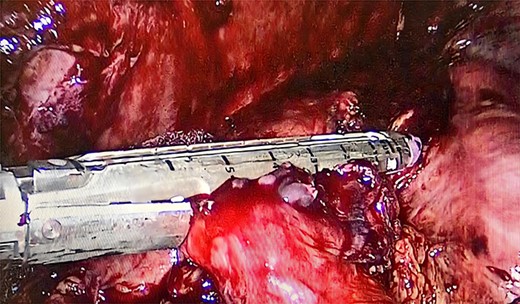
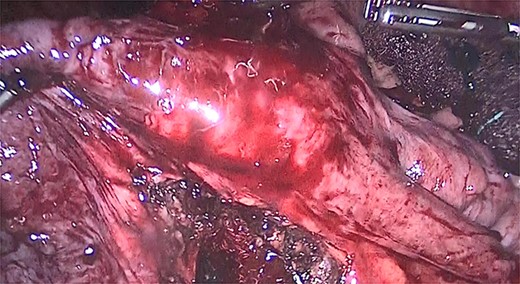
We performed adhesiolysis, used a harmonic scalpel to release intestinal loops and identified the gastric pouch (which was tight and measured < 15 cm long), remaining stomach, gastrojejunostomy and the common limb (∼200 cm), the total intestinal length was 600 cm (Fig. 2). We proceeded to make a new gastric pouch with a black staple (60 mm) and performed a gastro-gastro anastomosis with a purple staple (45 mm) (Fig. 3). The angle of Treitz was identified, and we found the jejunojejunostomy at 200 cm (Fig. 4). We later identified the gastroileal anastomosis, proceeded to resect that segment of 15 cm (Fig. 5) and the surgical procedure was completed.
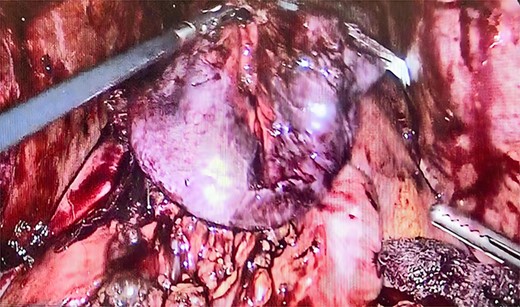
The patient had a postoperative period without complications. On Day 3, she resumed oral fluid feeding and gradually progressed. New laboratory test was performed and an improvement in hemoglobin of 12.2 g/dL, hematocrit of 36%, total bilirubin of 0.8 mg/dL, albumin of 3.9 g/dL was reported.
On Day 7, she was discharged after a weight gain of 3 kg, and a low protein diet was established. The patient had a total weight gain of 14 kg at 1 year after the surgery; today she is healthy, consumes a regular diet and has no complications.
DISCUSSION
Reversal surgery is rare so there are not many reported cases, in this patient it was a major challenge because she had other bariatric surgeries as background and her nutritional situation further complicated her treatment, so first she was stabilized so she could undergo a reversal surgery. Despite being a relatively new therapy, OAGB has to be thoroughly and critically examined to improve outcomes and address any possible issues. Concerns regarding the effects of the procedure after surgery have taken precedence over attempts to demonstrate its usefulness. Published results show a usually low early and late complication rate of 3 and 10% [4]. The YOMEGA trial determined that OAGB is not inferior to RYGB in terms of % excess BMI loss at 2 years. The procedure’s malabsorptive effect may have contributed to OAGB’s successful weight loss and metabolic results [7]. The reversal of the OAGB and RYGB are some challenging procedures because of the number of anastomoses and the clinical and nutritional situation in which the patients need the reversal. However, several authors have reported good results in patients who have undergone this treatment, both in short and long term, with remission of their complications [8, 9].
The inability to tolerate oral feeding and the rapid loss of considerable amounts of weight are linked to an increase in fatty acid synthesis, their conversion to ketones in the liver and the buildup of triglycerides that can lead to severe hepatic steatosis [5, 10, 11]. The viral hepatitis should be checked in patients in this age group because their symptoms can range from asymptomatic to fulminant hepatitis [12, 13].
The limitation of this case report is that, given the rarity of OAGB and RYGB reversal surgery, there are no guidelines for determining whether a patient requires reversal surgery. However, scientific evidence has shown that, in the presence of certain complications, reversal surgery causes weight gain and should be considered as a treatment [8, 14]. Another limitation of the case is that none of the first two surgeries were performed in our hospital. Despite the scant data, there are some authors who directly recommend reverting to normal anatomy instead of performing another type of bariatric surgery when complications such as severe malnutrition occur and this should be considered anytime a reversal surgery is planned [15].



