-
PDF
- Split View
-
Views
-
Cite
Cite
Takahiro Arima, Takayuki Tatebayashi, Sakashi Noji, Management of fulminating non-cardiogenic pulmonary edema following cardiac surgery, Journal of Surgical Case Reports, Volume 2023, Issue 1, January 2023, rjac625, https://doi.org/10.1093/jscr/rjac625
Close - Share Icon Share
Abstract
A malignant form of non-cardiogenic pulmonary edema (NCPE) that develops soon after the termination of cardiopulmonary bypass support during cardiac surgery is rarely encountered. It sometimes requires immediate management, including venovenous extracorporeal membrane oxygenation (VV-ECMO). In the first case, a 78-year-old female patient experienced fulminating NCPE after mitral valve plasty, which caused severe respiratory failure and hemodynamic instability due to a huge amount of sputum. In the second case, a 47-year-old male patient presented with right-sided unilateral pulmonary edema with a substantial amount of sputum after minimally invasive cardiac surgery for mitral valve repair. In both cases, VV-ECMO and aggressive fluid replacement were promptly initiated. The NCPE resolved on post-operative day 2, resulting in the successful termination of VV-ECMO. NCPE leads to lethal respiratory failure with multifactorial causes during cardiac surgery. However, as NCPE is potentially transient, immediate treatment comprising VV-ECMO and aggressive fluid replacement can improve clinical outcomes.
INTRODUCTION
Respiratory failure is considered to be one of the most serious complications of cardiac surgery. Non-cardiogenic pulmonary edema (NCPE) is a cause of post-operative respiratory failure. A malignant form of NCPE is a life-threatening condition that is difficult to treat successfully because of its rarity and lack of clinical experience. When mechanical ventilation cannot provide sufficient support, venovenous extracorporeal membrane oxygenation (VV-ECMO) is necessary. This case report aims to describe fulminating NCPE following cardiac surgery and its management, including VV-ECMO.
CASE SERIES
Case 1
A 78-year-old female patient with breathlessness and lethargy was admitted to the hospital. She had insulin-dependent diabetes mellitus, hypertension and chronic atrial fibrillation and was hospitalized for congestive heart failure a month prior. On admission, echocardiography revealed severe mitral regurgitation (MR) due to tethering of the mitral leaflets and moderate tricuspid regurgitation. She underwent mitral valve plasty (MVP), tricuspid annular repair, left atrial appendage closure and left atrial maze procedure using 32-mm Physio II and 32-mm tricuspid annuloplasty (TAP) rings (Edwards Lifesciences, Irvine, CA, USA) and Isolator Synergy ablation clamps (AtriCure Inc, Ohio, USA).
After terminating cardiopulmonary bypass (CPB), protamine sulfate was slowly injected peripherally for ~15 min. Meanwhile, the transfusion of packed red blood cells (RBCs) was initiated, and ~500 ml of pleural effusion in the right thoracic cavity was removed. Transesophageal echocardiography revealed no remarkable findings, including MR. However, ~40 min after CPB withdrawal, massive frothy sputum was expelled suddenly from the tracheal tube. The pulmonary artery pressure increased significantly from 15/8 to 45/30 mmHg, while the systemic blood pressure dropped to 60/40 mmHg due to fluid loss. Hence, fluid resuscitation and catecholamine administration were required to achieve hemodynamic stability. An estimated 164 ml blood loss was noted during this operation, and the in-out balance was +630 ml. Neither urticaria nor abnormal dermatological findings were observed. The operation time was 339 min, including an aortic cross-clamp time of 151 min and a CPB time of 211 min. The respiratory mode during CPB was 1-l airflow to prevent lung collapse.
In the intensive care unit, a high positive end-expiratory pressure (PEEP) was required at 12–16 cmH2O, with a peak inspiratory pressure of 30 cmH2O and FIO2 of 1.0. Nonetheless, arterial blood gas showed a pH of 6.99, pO2 of 77.7 mmHg and pCO2 of 107 mmHg. Meanwhile, cardiac function was comparatively maintained, with a cardiac index of ~2.4 l/min/m2. Chest radiography (CXR) showed bilateral consolidation as well as remarkable shrinkage of the cardiac silhouette compared to the preoperative CXR (Fig. 1), suggesting an extremely low left atrial pressure. Therefore, VV-ECMO (Capiox; Terumo Inc, Tokyo, Japan) was immediately initiated after placing a 21 Fr drainage cannula in the right atrium through the right femoral vein and a 15 Fr return cannula through the left femoral vein, guided by ultrasonography. Nafamostat mesilate was used for anticoagulation with a target-activated clotting time (ACT) of 180 s. Within 12 h post-operatively, the patient achieved hemodynamic stability with the administration of catecholamine and fluid replacement, including blood transfusion. The amount of yellowish sputum in the tracheal tube decreased significantly, reaching nearly 4000 ml in total. To compensate for the fluid and protein loss, 14 units (1680 ml) of FFP, 500 ml (100 g) of 20% albumin solution and 3000 ml of crystalloid solutions were intravenously administered over 12 h. No significant bleeding or fluid loss from the surgical site was observed.
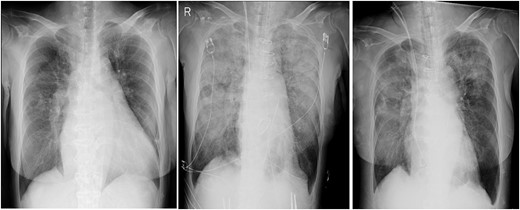
Chest radiographs obtained pre-operation (left), post-operation (middle) and 2 days post-operatively (right).
On post-operative day (POD) 2, VV-ECMO was discontinued after noting significant improvement with mechanical ventilation with PEEP at 12 cmH2O and FIO2 at 0.45. The ECMO duration was 39 h. Mechanical ventilatory support was terminated on POD 14. The patient required rehabilitation because of post-intensive care syndrome and was discharged on POD 70.
Case 2
A 47-year-old male patient was admitted to our hospital for infectious endocarditis and severe MR. He had chronic obstructive pulmonary disease, epilepsy and cerebral aneurysm. Echocardiography revealed severe MR due to the prolapse of both anterior and posterior leaflets. The patient underwent minimally invasive cardiac surgery (MICS) for mitral valve repair using a 32-mm Physio Flex (Edwards Lifesciences, Irvine, CA, USA).
After the termination of CPB and commencement of lung ventilation, frothy sputum was suddenly expelled from the right lumen of the double-lumen tracheal tube. At this point, neither blood transfusion nor protamine sulfate was provided. The thermodilution catheter showed a cardiac index of ~2.7 l/min/m2, and transesophageal echocardiography showed no significant findings. Fluid resuscitation involved 800 ml of autologous blood, 4 units (560 ml) of RBCs, 6 units (720 ml) of FFP and 500 ml (25 g) of 5% albumin solution to compensate for fluid loss through the frothy sputum. An estimated blood loss of 226 ml was noted during this operation, and the in-out balance was +5167 ml because of aggressive fluid resuscitation. The operation time was 717 min, including an aortic cross-clamp time of 194 min and a CPB time of 314 min. The respiratory mode during CPB was 2 l of oxygen flow to prevent lung collapse and achieve oxygenation.
Post-operative CXR showed unilateral consolidation (Fig. 2). Re-expansion pulmonary edema was suspected. Mechanical ventilation with a high PEEP of 12–14 cmH2O and a peak inspiratory pressure of 30 cmH2O was provided; nonetheless, there was no improvement in the respiratory condition, resulting in hypercapnia and severe acidosis with a pH of 7.06 and pCO2 of 94.4 mmHg. Thus, VV-ECMO was immediately initiated after placing a 21 Fr drainage cannula in the right atrium through the right femoral vein and a 15 Fr return cannula through the left femoral vein under fluoroscopic guidance. Unfractionated heparin was used for anticoagulation with a target ACT of 150 s, while being concerned about the risk of bleeding from the surgical site.
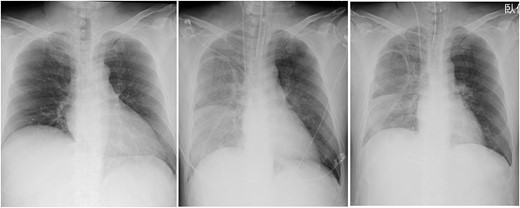
Chest radiographs obtained pre-operation (left), post-operation (middle) and 2 days post-operatively (right).
| Case . | . | Age (y) . | Sex . | Operation . | ECMO support (h) . | Canulation site . | Anticoagulation . | Survival at discharge . |
|---|---|---|---|---|---|---|---|---|
| 1–8 | Nakamura et al. [1] | 35–83, median: 64.5 | Male: 6, female: 2 | Conventional cardiac surgery | 17–260, median: 80.5 | Drainage cannula: femoral vein return cannula: opposite femoral vein or right jugular vein | ACT: 180–200 s | Two out of eight died |
| 9 | Vardas PN et al. [2] | 62 | Male | AVR, MVP, CABG | 72 | Dual lumen: right jugular vein | N/A | Yes |
| 10 | Bignami E et al. [3] | 66 | Male | MVP, TAP | 192 | Drainage cannula: right femoral vein return cannula: left jugular vein | N/A | Yes |
| 11, 12 | Takagaki M et al. [4] | 64 | N/A | Operation for MR | 1128 | N/A | No anticoagulation | Yes |
| 69 | N/A | Operation for MR | 96 | N/A | No anticoagulation | Yes | ||
| 13, 14 | Goyal S et al. [5] | 48 | Female | MVP (MICS) | 149 | Dual lumen: right jugular vein | N/A | Yes |
| 40 | Male | MVP (MICS) | 120 | Dual lumen: right jugular vein | N/A | Yes | ||
| 15 | Kitahara et al. [6] | 60 | Male | MVP (MICS) | 120 | Drainage cannula: right jugular vein return cannula: left femoral vein | N/A | Yes |
| Case . | . | Age (y) . | Sex . | Operation . | ECMO support (h) . | Canulation site . | Anticoagulation . | Survival at discharge . |
|---|---|---|---|---|---|---|---|---|
| 1–8 | Nakamura et al. [1] | 35–83, median: 64.5 | Male: 6, female: 2 | Conventional cardiac surgery | 17–260, median: 80.5 | Drainage cannula: femoral vein return cannula: opposite femoral vein or right jugular vein | ACT: 180–200 s | Two out of eight died |
| 9 | Vardas PN et al. [2] | 62 | Male | AVR, MVP, CABG | 72 | Dual lumen: right jugular vein | N/A | Yes |
| 10 | Bignami E et al. [3] | 66 | Male | MVP, TAP | 192 | Drainage cannula: right femoral vein return cannula: left jugular vein | N/A | Yes |
| 11, 12 | Takagaki M et al. [4] | 64 | N/A | Operation for MR | 1128 | N/A | No anticoagulation | Yes |
| 69 | N/A | Operation for MR | 96 | N/A | No anticoagulation | Yes | ||
| 13, 14 | Goyal S et al. [5] | 48 | Female | MVP (MICS) | 149 | Dual lumen: right jugular vein | N/A | Yes |
| 40 | Male | MVP (MICS) | 120 | Dual lumen: right jugular vein | N/A | Yes | ||
| 15 | Kitahara et al. [6] | 60 | Male | MVP (MICS) | 120 | Drainage cannula: right jugular vein return cannula: left femoral vein | N/A | Yes |
N/A, not applicable; AVR, aortic valve replacement; CABG, coronary artery bypass grafting.
| Case . | . | Age (y) . | Sex . | Operation . | ECMO support (h) . | Canulation site . | Anticoagulation . | Survival at discharge . |
|---|---|---|---|---|---|---|---|---|
| 1–8 | Nakamura et al. [1] | 35–83, median: 64.5 | Male: 6, female: 2 | Conventional cardiac surgery | 17–260, median: 80.5 | Drainage cannula: femoral vein return cannula: opposite femoral vein or right jugular vein | ACT: 180–200 s | Two out of eight died |
| 9 | Vardas PN et al. [2] | 62 | Male | AVR, MVP, CABG | 72 | Dual lumen: right jugular vein | N/A | Yes |
| 10 | Bignami E et al. [3] | 66 | Male | MVP, TAP | 192 | Drainage cannula: right femoral vein return cannula: left jugular vein | N/A | Yes |
| 11, 12 | Takagaki M et al. [4] | 64 | N/A | Operation for MR | 1128 | N/A | No anticoagulation | Yes |
| 69 | N/A | Operation for MR | 96 | N/A | No anticoagulation | Yes | ||
| 13, 14 | Goyal S et al. [5] | 48 | Female | MVP (MICS) | 149 | Dual lumen: right jugular vein | N/A | Yes |
| 40 | Male | MVP (MICS) | 120 | Dual lumen: right jugular vein | N/A | Yes | ||
| 15 | Kitahara et al. [6] | 60 | Male | MVP (MICS) | 120 | Drainage cannula: right jugular vein return cannula: left femoral vein | N/A | Yes |
| Case . | . | Age (y) . | Sex . | Operation . | ECMO support (h) . | Canulation site . | Anticoagulation . | Survival at discharge . |
|---|---|---|---|---|---|---|---|---|
| 1–8 | Nakamura et al. [1] | 35–83, median: 64.5 | Male: 6, female: 2 | Conventional cardiac surgery | 17–260, median: 80.5 | Drainage cannula: femoral vein return cannula: opposite femoral vein or right jugular vein | ACT: 180–200 s | Two out of eight died |
| 9 | Vardas PN et al. [2] | 62 | Male | AVR, MVP, CABG | 72 | Dual lumen: right jugular vein | N/A | Yes |
| 10 | Bignami E et al. [3] | 66 | Male | MVP, TAP | 192 | Drainage cannula: right femoral vein return cannula: left jugular vein | N/A | Yes |
| 11, 12 | Takagaki M et al. [4] | 64 | N/A | Operation for MR | 1128 | N/A | No anticoagulation | Yes |
| 69 | N/A | Operation for MR | 96 | N/A | No anticoagulation | Yes | ||
| 13, 14 | Goyal S et al. [5] | 48 | Female | MVP (MICS) | 149 | Dual lumen: right jugular vein | N/A | Yes |
| 40 | Male | MVP (MICS) | 120 | Dual lumen: right jugular vein | N/A | Yes | ||
| 15 | Kitahara et al. [6] | 60 | Male | MVP (MICS) | 120 | Drainage cannula: right jugular vein return cannula: left femoral vein | N/A | Yes |
N/A, not applicable; AVR, aortic valve replacement; CABG, coronary artery bypass grafting.
Two days post-operatively, VV-ECMO was terminated due to improvement in respiratory function, hypercapnia and acidosis. The amount of sputum also decreased remarkably with PEEP at 12 cmH2O. The ECMO duration was 41 h. Mechanical ventilatory support was terminated on POD 9. The patient was discharged on POD 48.
DISCUSSION
Post-operative NCPE significantly affects the clinical outcomes of cardiac surgery. It is often difficult to clarify the etiology of this challenging condition because NCPE is multifactorial. To our knowledge, cases of fulminating NCPE following cardiac surgery which require ECMO are associated with transfusion-related lung injury (TRALI) [7,8], re-expansion pulmonary edema [2,5,6] and protamine-induced pulmonary edema [9,10]. In Case 1, the main etiology of alveolar flooding was speculated to be TRALI, although re-expansion of the lungs and protamine administration were also possible causes because all these events occurred during the limited time frame soon after CPB termination. According to the new consensus of the TRALI definition, this case satisfied the criteria for TRALI type-1: acute onset, hypoxia, bilateral pulmonary edema, absence of left atrial hypertension and no risk factors for acute respiratory distress syndrome [11]. According to this consensus, the diagnosis of TRALI does not require the presence of cognate white blood cell antibodies. By contrast, in Case 2, re-expansion pulmonary edema was highly suspected as the main etiology because there were no other potential factors, such as blood transfusion and protamine administration, when frothy sputum was expelled through the tracheal tube. It has been reported that re-expansion of pulmonary edema often occurs after MICS, with an incidence rate of 2.1% [12], and in some cases, the patients were successfully treated with VV-ECMO [5, 6].
NCPE deteriorates rapidly following the appearance of frothy sputum and is sometimes life threatening. However, the NCPE is potentially temporary. In our cases, VV-ECMO support was commenced against hypoxia and/or severe acidosis due to hypercapnia within 2.0–2.5 h after the surgeries. The amount of frothy sputum decreased over time, resulting in a significant improvement in the respiratory condition, and VV-ECMO could be terminated on POD 2. These two cases suggest that immediately starting treatment with VV-ECMO can improve the outcome of fulminating NCPE.
Other cases treated with VV-ECMO within 24 h post-operatively for respiratory failure are reviewed in Table 1 [1–6]. The following observations were made based on the cases reviewed. First, previous surgeries were categorized into conventional cardiac surgery and MICS. Second, the ECMO duration in other cases was much longer than those in our cases. This may be due to the difference in the safety margin of ventilator settings when terminating VV-ECMO. In fact, we discontinued VV-ECMO with PEEP at ~12–14 cmH2O and FIO2 at 0.45, which may not be an acceptable setting for other clinicians. Considering the risk of bleeding due to anticoagulation, we prioritized the earlier termination of VV-ECMO. Another possible reason is the difference in patients’ characteristics, especially pre-existing respiratory diseases; however, there is limited detailed information about preoperative conditions. Third, in terms of cannulation sites, we chose bilateral femoral veins because the right jugular vein was already occupied by other catheters. As reported in other cases, there were many choices, including dual lumen cannula through the right jugular vein. The most important point is to avoid recirculation related to the location of the tips of each cannula. A drainage cannula was placed at the right atrium via the right femoral vein and a return cannula was placed at the left common iliac vein via the left femoral vein under ultrasonographic or fluoroscopic guidance.
Regarding anticoagulation therapy, the risk of surgical site bleeding soon after cardiac surgery is a major concern. The choice of anticoagulation therapy may potentially determine the outcome of patients. Heparin is the most common choice, whereas nafamostat mesilate may be an alternative in cases involving a high risk of bleeding events. It has been reported that when using nafamostat mesilate, which has a short half-life, activated coagulation time and partial thromboplastin time of the patients’ blood were lower than those of ECMO circuits [13]. Although nafamostat mesilate was potentially able to reduce the bleeding risk, it increased the risk of hemorrhagic events in another [14]. As these studies were retrospective, further research is required. Takagaki et al. reported that post-cardiotomy VV-ECMO without heparization could facilitate patient rescue, with a survival rate of four out of eight cases [4]. In another study, a target activated partial thromboplastin time of 35–40 s under heparization did not cause any major thromboembolic complications in 36 patients with VV-ECMO [15]. The anticoagulation strategy remains controversial; therefore, further studies are required.
CONCLUSIONS
NCPE leads to lethal respiratory failure with multifactorial etiologies during cardiac surgery. However, as NCPE is potentially transient, immediate treatment comprising VV-ECMO, and aggressive fluid replacement can improve clinical outcomes.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



