-
PDF
- Split View
-
Views
-
Cite
Cite
Tulio Brasileiro Silva Pacheco, Andre Camara Matoso Chacon, Jasmine Brite, Amir H Sohail, Manesh Kumar Gangwani, Rafael D Malgor, Jun Levine, Gutenberg do Amaral Gurgel, Nutcracker phenomenon secondary to superior mesenteric artery syndrome, Journal of Surgical Case Reports, Volume 2023, Issue 1, January 2023, rjac622, https://doi.org/10.1093/jscr/rjac622
Close - Share Icon Share
Abstract
Superior mesenteric artery (SMA) syndrome, compression of the duodenum due to a decreased angle between the aorta and SMA, has a wide range of clinical presentations making it difficult to diagnose. Compression of the left renal vein is known as Nutcracker syndrome. We present the case of a 26-year-old male with a delayed diagnosis of SMA syndrome and Nutcracker phenomenon due to the patient’s history of chronic pancreatitis. As a result of his obstruction and aspiration pneumonia, he was diagnosed with septic shock. The patient was treated for septic shock and maintained on an enteric diet with improvement in the body mass index and complete resolution of SMA syndrome symptoms.
INTRODUCTION
Superior mesenteric artery (SMA) syndrome, also known as Wilkie Syndrome, is a rare condition that if not recognized and treated early can become life-threatening. The culprit and most important risk factor to be identified during the anamnesis is rapid weight loss followed by GI obstructive symptoms [1, 2].
Fundamentally, a reduction in the posterior aspect of the mesenteric fat pad, which circumferentially encases the root of the SMA, leads to extrinsic compression of the third portion (retroperitoneal horizontal segment) of the duodenum [3]. Therefore, the etiologies related to SMA syndrome are those associated with weight loss, including malabsorption, malignancy, AIDS, bariatric surgery, burns, spinal cord injury, paraplegia, drug abuse, prolonged bed rest, anorexia nervosa and thyrotoxicosis [2, 4, 5].
In rare cases, the left renal vein might be affected by the acute reduced angle between the SMA and aorta. This phenomenon is called Nutcracker syndrome in which patients experience hematuria, proteinuria and/or left flank or back pain [6].
We present the case of a 26-year-old male who had a delayed diagnosis of SMA syndrome and Nutcracker phenomenon due to a medical history of chronic pancreatitis.
CASE REPORT
A 26-year-old male was admitted to the emergency room with gastrointestinal obstructive symptoms of 24 hours duration with associated aspiration pneumonia and septic shock. Past medical history was significant for multiple previous admissions due to chronic pancreatitis secondary to alcoholism, Type 1 diabetes mellitus, schizophrenia and epilepsy. Past surgical history was unremarkable and family history was unknown.
Upon admission, the patient was severely malnourished with a body mass index (BMI) of 15.7 kg/m2. On physical exam, his abdomen was softly distended and tympanic but non-tender. On chest auscultation, the patient had diminished breath sounds at the lower base of the right lung. He was transferred to the intensive care unit due to refractory shock and started on vasopressors and broad-spectrum antibiotics. A nasogastric tube was placed with an output of approximately 1.8 L of bilious content. After hemodynamic stabilization, computed tomography (CT) scan of the abdomen was performed, which revealed a distended stomach and first and second portion of duodenum (Fig. 1).
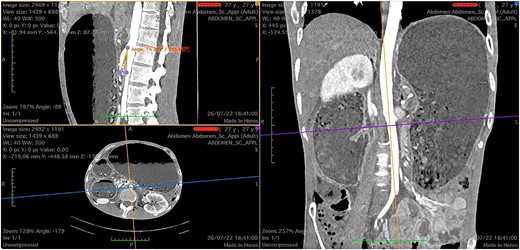
Computed tomography images showing a narrow aortomesenteric angle of 14.3° (sagittal section) and a massively dilated stomach, as seen on coronal and axial sections.
There was extrinsic compression of third portion of the duodenum by the SMA (Fig. 2).
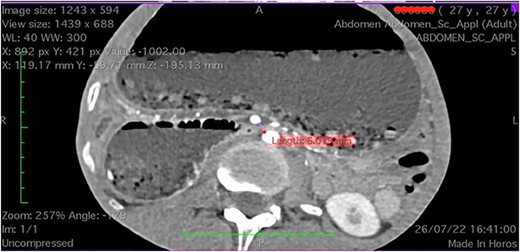
Computed tomography axial section showing a dilated stomach resulting from compression of third portion of duodenum.
A diagnosis of SMA syndrome associated with extrinsic compression of the left renal vein was made. Esophagogastroduodenoscopy was performed revealing Candida esophagitis (for which antibiotic treatment was initiated), and a post-obstruction Dobhoff tube was placed.
The patient improved and was subsequently discharged with enteric nutrition and oral antibiotics. During the entire hospitalization, the patient denied any history of hematuria, decreased urine output, and flank or low back pain.
At a 2-month follow up, the patient achieved a BMI of 18 kg/m2 and resolution of gastrointestinal obstructive symptoms with no recurrence of abdominal pain. Again, the patient denied any hematuria, oliguria, and flank or low back pain. CT imaging was avoided at this time as symptomatology had resolved.
DISCUSSION
First reported by Carl Von Ronkitansky in 1842 and later categorized by Wilkie [1], SMA syndrome is a rare condition with a prevalence of approximately 0.3% and a female predilection. The peak incidence is usually between 10 and 39 years of age [3].
SMA syndrome has a broad spectrum of clinical manifestations. It can present acutely, especially after rapid weight loss, with nausea and bilious emesis or chronically with mild epigastric pain, tympanism and early satiety due to the gastric distention. Comorbidities can disguise the ultimate diagnosis leading to perpetuation and chronicity of the condition. In our case, the patient’s history of chronic pancreatitis due to alcoholism, leading to multiple hospitalizations, caused a significant delay in the diagnosis of SMA syndrome. As a result, the prolonged obstruction of the patient’s stomach and duodenum caused massive distention leading to complications such as aspiration pneumonia [7].
Radiological findings are pivotal for the final diagnosis of SMA syndrome. A normal SMA and aorta artery angle ranges from 38 to 65°. Radiological criteria of SMA syndrome includes duodenal obstruction with rapid cutoff in the third part, aortomesenteric artery angle ≤25°, aortomesenteric distance ≤8 mm and abnormally high fixation of duodenum by the ligament of Treitz or low SMA origin [8].
Nutcracker syndrome results from a reduced angle between the SMA and the aorta causing external compression of the left renal vein [9]. Patients typically present with hematuria, decreased urine output and back/flank pain. Rarely, left splenorenal shunts form because of the prolonged proximal stenosis of the left renal vein. The shunt also serves as collateral circulation to the left gonadal vein [10]. The terminology of Nutcracker phenomenon/anatomy is used when imaging findings are present in an asymptomatic patient [11]. Although our patient presented with remarkable obstruction of the left renal vein, he was asymptomatic.
Even though a variety of radiological studies are part of the armamentarium used to diagnose SMA syndrome, including CT, MRI, ultrasound, Doppler ultrasound and conventional angiography, diagnostic delay is still one of the reasons SMA syndrome can become life-threatening [12]. Unfortunately, in this case, the patient’s comorbidity (chronic pancreatitis) and social history (alcoholism) also contributed to the delay in diagnosis. As a consequence, the patient presented critically ill with septic shock secondary to aspiration pneumonia in a cachectic state requiring intensive care. In addition, even though the Nutcracker anatomy/phenomenon was identified on imaging, the Nutcracker syndrome could not be confirmed as symptomatology was clouded by chronic pancreatitis symptoms [11].
The goal in SMA syndrome management is to restore the mesenteric fat pad between the aorta and SMA aiming to increase the angle between the aforementioned vessels to relieve the obstruction. National Institute for Health and Care Excellence guidelines for assessing nutritional status in adults on enteric nutrition and TPN may be used. Surgical options for SMA syndrome, including Strong’s procedure, feeding jejunostomy, gastrojejunostomy or duodenojejunostomy may also be considered, especially in patients with poor response or feasibility for oral feeding or TPN [13]. In our case, the patient had a fast recovery from the septic shock and tolerated enteric nutrition without any complications.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
DATA AVAILABILITY
Since this is a case report all relevant data were included. However, if further information is required, it can be provided upon request.



