-
PDF
- Split View
-
Views
-
Cite
Cite
Joseph de Nanassy, David Mack, Marcos Bettolli, Consolato M Sergi, Diverticular disease in a pediatric patient with Crohn’s disease mimicking a perforated post-appendectomy appendiceal stump, Journal of Surgical Case Reports, Volume 2022, Issue 8, August 2022, rjac355, https://doi.org/10.1093/jscr/rjac355
Close - Share Icon Share
Abstract
We present the finding of a diverticulum in the colonic wall of the cecum, arising in the context of ileocecal stricture in a child with Crohn disease mimicking a post-appendectomy perforated appendiceal stump. To our knowledge, a non-Meckel diverticulum in a pediatric patient with Crohn disease has not yet been reported and we examine the mechanics behind it. According to the Laplace Law, the pressure inside a container with curved walls is inversely proportional to its radius. A diverticulum forms at the point of maximum stricture and at the locus of least resistance (weakness) in the bowel wall due to the inflammatory bowel disease. The long-time interval between diagnosis of ileocecal stricture and surgery (9 months) is important to allow the formation of this diverticulum. Continued follow-up in adulthood is warranted due to an increased risk of intestinal diverticular disease and neoplasms in patients with Crohn disease.
INTRODUCTION
The inflammatory bowel disease—Crohn disease (IBD-CD) has been associated occasionally with diverticulosis of small bowel and adenocarcinoma in adults [1–3], but a non-Meckel diverticulum in a pediatric patient with IBD-CD has not yet been reported to the best of our knowledge. Pediatric IBD-CD reveals on the same features of transmural inflammation, ulceration, lymphoid aggregates and granulomas, as identified in adults [4]. An increased epithelial gap has been detected in IBD and has been recognized as a predisposing factor for the inflammation [5, 6]. We present the unusual case of a diverticulum of the colonic wall in the cecum, arising in the context of severe ileocecal stricture in IBD-CD.
CASE REPORT
A 15-year-old male was diagnosed with IBD-CD. The fecal calprotectin level was 2170 μg/g, and C-reactive protein was 16.4 mg/L. The white blood cell count was 7.1 x 103 (neutrophils of 5.0 x 103). Testing for viruses was non-contributory. The stool was negative bacteria and pathogenic parasites. Magnetic resonance imaging (MRI) showed features in keeping with IBD-CD of the terminal ileum and cecum. Imaging studies a year later revealed a small posterior–superior extension from the ileocecal junction, which was interpreted as being the appendix, despite appendectomy having been performed several years earlier. Colonoscopy identified marked narrowing of <0.3 cm (Ø) at the ileocecal valve. Initial medical therapy for treating IBD-CD was unsuccessful in resolving the fibrostenotic stricturing. Ileocecal resection was eventually performed 9 months later.
Gross examination of the resected ileocecal specimen revealed an internal diameter of ~0.4 cm at the stenotic ileocecal valve. In addition, there was a cystic structure adjacent to the ileocecal valve with a smooth internal aspect and in direct continuity with the lumen of the bowel through an opening of 0.3 cm in diameter (Fig. 1A). The tubular diverticulum (4 x 2.5 x 2.5 cm) was filled with fecal material. Microscopy of the ileocecal region showed features of IBD-CD. In addition, there was a cystically dilated diverticulum adjacent to the colonic wall, arising from the area of the ileocecal valve, extending in a superior and posterior direction, and lined by flattened colonic mucosa without evidence of IBD-CD (Fig. 1B). Our interpretation is that of a colonic diverticulum arising at a point of localized weakness in the bowel wall. This colonic diverticulum would have arisen in the context of markedly increased intraluminal pressure on the bowel wall secondary to long-standing stenosis at the ileocecal junction. The stenosis at the ileocecal valve and the opening of the diverticulum were of about equal diameter (0.3–0.4 cm). No other diverticula were identified.
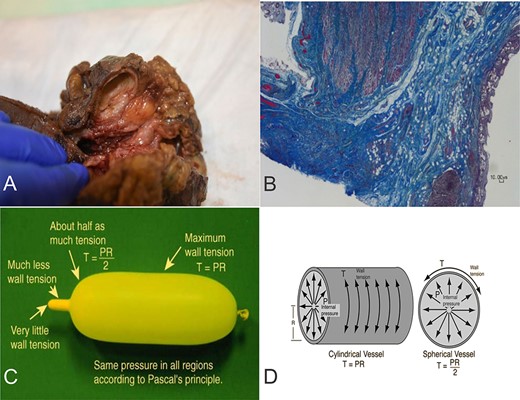
(A) Diverticulum at the junction of terminal ileum (right) and cecum (left). (B) Ileocecal muscle (left) and diverticulum with flattened colonic mucosa (right). Masson trichrome. X2 magnification. (C-D) Laplace’s Law: the pressure inside a container with curved walls is inversely proportional to its radius. Figure 1C and 1D are reproduced from "Hyperphysics" (http://hyperphysics.phy-astr.gsu.edu/hbase/ptens.html) with permission received from Dr. C. R. Nave, HyperPhysics Project, Department of Physics and Astronomy, Georgia State University, Atlanta, GA 30303, United States.
DISCUSSION
We describe a hitherto unreported occurrence of a colonic diverticulum arising at the ileocecal junction in severe stenosing IBD-CD of several months’ duration in a 15-year-old male. Eventual ileocecal surgical resection was carried out when medical management failed to relieve the clinical symptoms associated with the stricture. A finding of interest was the presence of a colonic diverticulum of several centimeters in dimension, arising in the region of the ileocecal valve, and containing fecal material. The colonic lining of the diverticulum did not show gross or microscopic features of IBD-CD.
Our assessment of this diverticulum is that of a secondary outpouching from the colonic wall at a point of maximal stenotic stricture in the setting of severe IBD-CD. The clinical context of prolonged ileocecal stricture of many months’ duration until surgical resection provided the conditions for an outpouching of such a relatively large size to form.
Differential diagnostic considerations for an ileocecal diverticulum include a duplication cyst, intussusception, a Meckel diverticulum, a focal neoplastic mass, localized inflammation in the bowel wall, appendicitis and perforated appendiceal stump among others. A duplication cyst of the colonic wall would occur at the mesenteric border of the main bowel segment, unlike the lesion in our case, which arose at a point of anatomical weakness in the muscularis propria layers in the region of the ileocecal valve. The muscularis propria layers of an intramural duplication cyst and those of the adjacent main bowel segment would be of about equal thickness when merging with each other, as opposed to a thinner muscularis propria layer in the diverticulum of our case being continuous with the markedly thickened muscularis propria layers of the cecum at the ileocecal junction. An extramural duplication cyst would have its own muscularis propria layer, separate from that of the main bowel wall. In general, duplication cysts tend to be of a larger size. Intussuscepted bowel segments reveal the presence of a double bowel wall on imaging studies, which was not demonstrated on several occasions of MRI in our patient. In addition, intussusception tends to occur in a younger age group. The site of the diverticulum in the case reported by us is in the wrong anatomical location for a Meckel diverticulum, which tends to be found at about 2 feet proximal of the ileocecal valve. A Meckel diverticulum might also contain heterotopic mucosa, which our specimen did not have. No neoplastic process was identified in our specimen. Finally, appendicitis would not have been recognized in our patient who had undergone appendectomy. A small residual appendiceal stump at the appendix base was separate from the diverticulum located at the ileocecal junction and not involved by the inflammatory process.
The first reports of IBD-CD and small bowel diverticular disease are rarely reported [7]. In 1998, Gledhill et al. [8] reported 11 patients with diverticulosis and inflammatory bowel disease and argued that diverticulosis and/or diverticulitis may be linked to the inflammatory response of IBD-CD. According to the Law of Laplace, the pressure inside an inflated container with a curved surface is inversely proportional to its radius [Pressure = (2 x Wall Thickness x Wall Tension) /Radius], which would explain the formation of the diverticulum close to the point of maximum stricture (Fig. 1C and D). The length of time between diagnosis of the severe stricture and eventual surgery (9 months) likely contributed to the formation of this unique colonic wall diverticulum [9, 10].
AUTHORS’ CONTRIBUTIONS
All authors contributed to the draft, collection of illustrative material and final approval of the manuscript.
ACKNOWLEDGMENTS
We are grateful for the generosity of the Children’s Hospital of Eastern Ontario. The funders had no role in study design, data collection, analysis, decision to publish or manuscript preparation. We are very grateful to Dr. C. R. Nave, HyperPhysics Project, Department of Physics and Astronomy, Georgia State University, Atlanta, GA, United States, for the possibility to reproduce Figures 1C and 1D.
DATA AVAILABILITY
Data are contained within the article. No new data were created or analyzed in this study. Data sharing is not applicable to this article. Anonymity was preserved in the publication of this case report.
CONFLICT OF INTEREST STATEMENT
The senior author has been involved in receiving honoraria, giving expert testimony, and receiving grants and royalties from publishers (Springer, Elsevier, Nova Science publishers).
References
- appendectomy
- crohn's disease
- inflammatory bowel disease
- adult
- asthenia
- child
- constriction, pathologic
- diverticulum
- follow-up
- intestines
- pediatrics
- radius
- surgical procedures, operative
- cecum
- diagnosis
- meckel's diverticulum
- neoplasms
- surgery specialty
- laplace law
- colonic wall
- appendiceal stump
- diverticular diseases



