-
PDF
- Split View
-
Views
-
Cite
Cite
Konstantinos Botsakis, Paraskevi Trakosari, Michail Papamichail, Sofia Petsa-Poutouri, Chariklia Kouvidou, Marina Paraskevopoulou, Dimitrios Chasiotis, Vassilios Vougas, Granular cell tumor of distal bile duct treated with Whipple’s procedure, Journal of Surgical Case Reports, Volume 2022, Issue 6, June 2022, rjac293, https://doi.org/10.1093/jscr/rjac293
Close - Share Icon Share
Abstract
Granular cell tumors (GCTs) are rare benign neoplasms that are most often located in the soft tissues of the extremities and chest wall. Malignant GCTs have also been reported. GCTs of the biliary tract are extremely rare, uncommon non-epithelial benign neoplasms that cause focal thickening of bile duct wall without mucosal invasion. They consist of polygonal cells with granular appearance and stain positive in S-100 protein, indicating a neural (Schwann cell) origin. We report our experience of a 57-year-old Caucasian woman who presented with obstructing jaundice due to a distal bile duct stricture highly suspicious of cholangiocarcinoma. A Whipple’s procedure was successfully performed, and the final pathology revealed a benign GCT of the distal bile duct. Whipple’s is an extremely radical procedure for such benign lesions and additional investigations, such as cytology sample, may result in a less aggressive approach as those tumors grow slowly and do not metastasize.
INTRODUCTION
Granular cell tumors (GCTs) are uncommon soft tissue neoplasms that have an indolent course and can occur in any organ but more often are located in the skin, tongue and subcutaneous tissues of the extremities and chest wall [1]. They can be single or multifocal and can affect patients of all age groups but are mostly seen between 40 and 60 years of age [1]. Five to eleven percent of all cases are found in the gastrointestinal tract, but <1% originate in the bile ducts and gallbladder and >80 cases have been reported so far [2]. Despite its rarity, this neoplasm is the most common non-epithelial tumor of the extrahepatic biliary tract [2]. GCTs have generally a distinct histological morphology composed of polygonal cells with granular appearance and stain positive in S-100 protein, indicating a neural (Schwann cell) origin [3].
Patients with GCTs of the biliary tract usually present with nonspecific abdominal pain and obstructive jaundice, but incidental finding on imaging of asymptomatic patients is not uncommon [4]. Imaging reveals a mass or stricture in the extrahepatic biliary tree with associated proximal dilatation which is often difficult to differentiate, and confirmation of diagnosis usually follows surgery [5]. However, cytology from endoscopic brushing may be helpful in some cases with large tumors [2, 6].
We present a case of a 57-year-old Caucasian woman with distal bile duct GCT which was treated successfully with Whipple’s procedure.
CASE REPORT
A 57-year-old Caucasian woman presented with a 2-week history of mild abdominal pain, jaundice, generalized pruritus, stool discoloration and dark urine. She had deranged liver function tests with total bilirubin 30.5 mg/dl alkaline phosphatase 729 U/l, SGOT 222 U/l, SGPT 291 U/l and γ-GT 837 U/l. The tumor marker Ca19–9 was within normal limits. Imaging revealed intrahepatic and extrahepatic bile duct dilatation with abnormal tapering at the distal end of common bile duct, without evidence of calculi or dilatation of pancreatic duct (Fig. 1). The patient had an unsuccessful ERCP due to difficult cannulation, which was followed by percutaneous transhepatic cholangiography (PTC) with external stent insertion to decompress biliary system. Brushing cytology was inconclusive (Fig. 2). Based on these findings, a diagnosis of distal cholangiocarcinoma was suspected. Further staging did not reveal any metastatic disease. The patient underwent Whipple’s procedure without any complications and was discharged on post-operative day 16 in a very good condition.
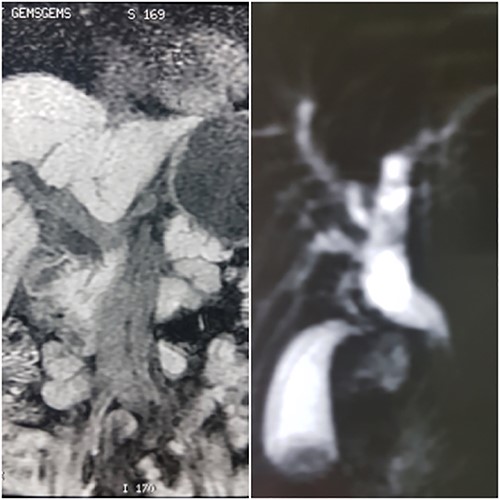
MRCP showing dilation of common bile duct extending intrahepatically.
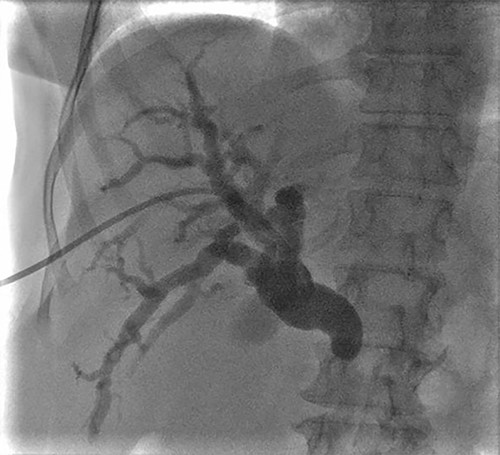
PTC showing dilation of intrahepatic biliary tree with abnormal tapering in the mid bile duct and lack of contrast filling distally.
Specimen’s gross examination revealed a focal dilatation of distal end of the bile duct (maximum diameter: 1 cm) with thickened wall but without any mucosa’s ulceration. Microscopic examination showed the presence of a neoplasm consisting of clusters of large ovoid and round cells with granular cytoplasm and small uniform hyperchromatic nucleus extending to the chorium without invading mucosal surface. No mitotic activity or necrosis was noted. There were further plenty of epithelioid and spindle cells in nests and sheets with abundant granular eosinophilic cytoplasm and bland nucleus. All the above findings were suggestive of a benign GCT of the distal bile duct. Additionally, the tumor stained positive for S-100 protein, inhibin and CD68 and stained negative for desmin, actin and CD117, and the Ki-67 index was ~1% (Fig. 3). The resection margins were free of tumor and there were 12 regional lymph nodes resected, which showed features of reactive lymphadenitis.
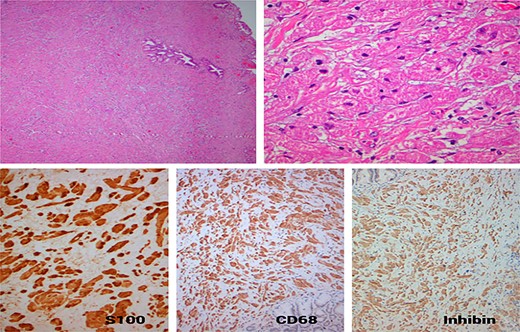
(A) Neoplastic growth of a poorly defined tumor with diffuse architecture in the fibromascular layer of the bile duct, with no signs of mucosal invasion or intraluminal extension (HE ×40); (B) the tumor is composed of polygonal cells with mild nuclear atypia and abundant granular eosinophilic cytoplasm separated by thin collagen bundles (HE ×400); (C) the tumor cells exhibit strong nuclear and diffuse cytoplasmic expression S100 protein (×200); (D) strong and diffuse cytoplasmic expression of CD68 (×100); (E) strong and diffuse cytoplasmic expression of Inhibin (×100).
The patient is currently followed up annually and has shown no evidence of recurrence 53 months after surgery.
DISCUSSION
GCTs originating in the biliary system was first described by Coggins in 1952 [7]. They are most often seen in the extra hepatic biliary tract (>80%), but cases involving cystic duct, gallbladder, intrapancreatic and intrahepatic bile ducts have also been reported [2, 8]. The incidence is higher in young black women with a mean age of 34 years [6]. The range of tumor size is between 0.5 and 4 cm, and a tendency of tumor growing in areas where bile ducts bifurcate (e.g. common bile duct and cystic duct or right and left hepatic ducts) has been described [5]. Multiple tumors within the biliary system can occur, however, in the majority of cases, single tumor is most likely to be found [8]. Patients with simultaneous biliary and skin and gastric GCTs have also been reported [3].
Microscopically GCTs consist of polygonal eosinophilic cells with small vesicular nuclei and abundant granules with positive periodic acid-Schiff staining [9]. The cells appear to lie in clusters or sheets within the nerves and surrounding soft tissue [6]. The granular appearance of the cytoplasm is related to the lysosome-like structures containing myelin, indicating a neural origin [3]. In addition, there is strong positivity with S-100 protein and other Schwann-cell-related antigens [3]. Mitoses and areas of necrosis are uncommon. All of the above were evident in our case in addition to the fact that there was no mucosal involvement but only thickening of the fibromuscular layer of the bile duct as described in other studies [10].
Despite the fact that tumor does not invade intraluminally, it causes obstruction from focal thickening of bile duct wall, and patients often present with jaundice and proximal dilatation of the biliary system [4]. In this scenario, exclusion of cholangiocarcinoma is difficult and definitive diagnosis usually follows surgery. In our case, malignant stricture of distal bile duct was highly suspected and surgery was radical. Hoda et al. reported two cases where cytology after endoscopic brushing showed the presence of granular cells on two patients with hilar and distal bile duct stricture. It remains unclear if this was helpful to differentiate the lesion and to prompt less aggressive management as both patients were treated with radical surgery [6]. Studies have shown that GCT cytology sample from other tissues (breast, skin, soft tissues, etc.) may have potential in identifying granular cells if the material is sufficient or if the surface epithelium is disrupted [6].
Only a small percentage (2%) of extra biliary GCTs were reported to be malignant, and histologic features of malignancy includes increased mitoses with necrosis, prominent spindling of cells and pleomorphic nuclei with prominent nucleoli [1, 9]. Quinn et al. reported a case of a 56-year-old patient with an infiltrative bile duct tumor who underwent resection of extrahepatic biliary tree. Histology showed a GCT with malignant features [11]. Multifocal intrahepatic GCT, which resulted in cholestasis, has been reported in two studies [8, 12]. The diffuse appearance of the tumor inside the liver was not suggestive of malignancy, as in both cases, final histology showed benign GCT. Granular cells were found in neural tissue along the portal triads in a pattern causing multiple small segmental strictures.
In conclusion, GCTs of the biliary tract are uncommon non-epithelial benign neoplasms, which are difficult to suspect because despite of lack of infiltration inside the bile ducts. Patients often present with obstruction from focal wall thickening, and even after extensive workup, malignancy cannot be excluded without definitive histology. Cytology from brushing may be helpful if a sufficient sample can be obtained and potentially may result in a less aggressive approach as those tumors grow slowly and do not metastasize.



