-
PDF
- Split View
-
Views
-
Cite
Cite
Aakash A Trivedi, James Y Yang, Jackie Johnston, Alan Sori, Septic shock due to candida and disseminated herpes simplex virus-1 (HSV1) after elective spinal surgery in an immunocompromised patient with chronic HSV1 infection, Journal of Surgical Case Reports, Volume 2022, Issue 6, June 2022, rjac273, https://doi.org/10.1093/jscr/rjac273
Close - Share Icon Share
Abstract
Herpes simplex virus is an infection that can result in a variety of symptoms ranging from blistering or ulcers to severe, systemic manifestations. We report a case of patient who underwent elective spinal surgery and developed invasive herpes as well as candidiasis postoperatively without any direct evidence of immunosuppression.
INTRODUCTION
Herpes simplex virus-1 (HSV1) affects 3.7 billion people under the age of 50 (67% of global population) worldwide. HSV1 is typically transmitted by oral but can also be transmitted through genital contact. Although often asymptomatic, HSV1 can cause painful ulcers ranging in severity [1]. Medications can reduce severity and frequency of symptoms; however, there is no known cure. Severe manifestations of HSV1 include encephalitis, hepatitis, pneumonitis, and esophagitis [2]. Once infected, HSV1 is a lifelong infection with the potential for recurrence. Immunocompromised hosts may experience more frequent and severe symptoms due to a of lack cellular immunity.
CASE REPORT
A 67-year-old Caucasian female was admitted to the intensive care unit (ICU) following elective posterior spinal instrumentation and fusion with laminectomy due to concerns for postoperative respiratory distress. Medical history of the patient included chronic back pain, obesity, hypertension, gastritis, HSV1 and tobacco use. Prior to surgery, the patient completed six courses of prednisone, 50 mg daily for 5 days over a 3-month period for chronic back pain.
Postoperative day (POD) 1, she desaturated to SpO2 of 85%. Oxygenation improved with continuous positive airway pressure. Chest X-ray was notable for bilateral lower lobe infiltrates. On the day of surgery, a glucocorticoid taper with dexamethasone and hydrocortisone was initiated. In addition, the patient received a one time dose of hydrocortisone of 100 mg. The patient received dexamethasone 10 mg twice daily for 1 day, 8 mg twice daily for 1 day and 6 mg once during POD 1–2. On POD 3, the patient was transitioned to hydrocortisone 50 mg every 6 h for 1 day, followed by hydrocortisone 25 mg every 6 h for 1 day.
On POD 2, the patient developed acute kidney injury. Blood cultures were ordered due to febrile episodes and vancomycin and piperacillin–tazobactam were initiated. On POD 5, she became hemodynamically unstable with hypercapnic respiratory failure. She was intubated and started on norepinephrine and hemodialysis. On POD 6, the white blood cell count doubled and procalcitonin was elevated. Repeat cultures were sent and antimicrobials were broadened to vancomycin and meropenem. Chest X-ray was concerning for acute respiratory distress syndrome (ARDS). Blood, urine and sputum cultures grew Candida albicans. Fluconazole was initiated.
Due to concerns for immunosuppression in the setting of glucocorticoid use and candedemia, additional testing was ordered. A β-D-Glucan was not performed as patient’s serum triglycerides were > 700 mg/dL. Sputum was negative for Pneumocystis jirovecii. Immunostaining showed many nuclear and cytoplasmic cells that were positive for HSV. Disseminated HSV1 infection was confirmed with DNA PCR. Acyclovir was initiated. Family reported that she had history of oral HSV1 and experienced recurrent oral infections.
On POD 15, the patient was noted to have bloody output from the orogastric tube and esophagogastroduodenoscopy was performed. Diffuse esophageal and gastric ulcerations were noted, consistent with HSV esophagitis (Figs 1 and 2). The patient began improving clinically. Vasopressors were discontinued and patient was extubated the following morning. She was transferred out of the ICU on POD 28 with improvement of renal function and discontinuation of dialysis. Patient was discharged on POD 39.
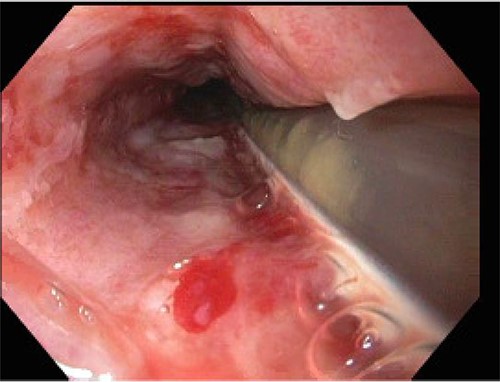
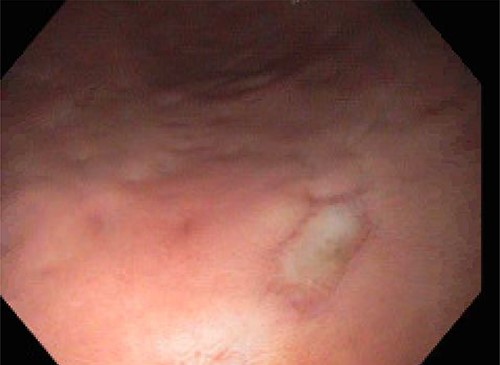
DISCUSSION
C. albicans has been described as an opportunistic infection occurring in immunocompromised hosts. Mortality is reported to be as high as 40% with disseminated candidiasis in immunocompromised individuals. Risk factors for disseminated candidiasis include malignancy, immunosuppressive factors such as corticosteroids, diabetes, infection and poor lifestyle habits such as chronic tobacco, alcohol and drug abuse [3]. As mentioned, our patient had completed multiple courses of prednisone therapy over the 3-month period preceding her elective surgery and received additional glucocorticoids postoperatively. Although our patient fully recovered after the initiation of appropriate therapy, this is a stark comparison to recent studies showing up to an 85.9% mortality in immunocompromised patients with septic shock due to invasive Candida spp. infections with prolonged ICU stay [4].
HSV1 infections usually cause ulcerated lesions. HSV viremia has been reported in up to 25% of immunocompetent patients with primary HSV infections [5]. Overall, the incidence of HSV1 reactivation in critically ill patients with septic shock ranges from 32 to 68% [6]. HSV1 esophagitis is a well-known complication as the esophagus is the most commonly involved visceral organ. HSV esophagitis is usually found in patients with acquired immunodeficiency syndrome, malignancies, those receiving immunosuppressive therapy or terminally ill [7]. There is no clearly defining link between preoperative hydrocortisone use over a short period of time and significant immunosuppression. Currently, there is no consensus on the risk of reactivation of cutaneous HSV1 progressing into disseminated viremia. The possible reasons for our patient include immunosuppression, spinal surgery in an obese patient, the development of ARDS or the preceding fungemia. ACS-NSQIP data from 2011 to 2014 identified that 3.7% of patients who underwent elective posterior lumbar fusion were on chronic glucocorticoids [8]. As previously mentioned, 67% of global population is estimated to be affected by HSV. This case demonstrates that the possibility of disseminated viral infection must be considered even after elective spinal cases.
CONCLUSION
HSV1 can present with severe symptoms in immunocompromised patients. These manifestations can stem from a variety of etiologies including patient risk factors, clinical condition, recent surgery and concomitant infection. This is one of the first descriptions in literature describing a patient undergoing elective spinal surgery whose postoperative course was complicated by disseminated HSV and invasive candedemia. In our patient, we believe that the viral and fungal sepsis stemmed from a combination of obesity, surgery, preceding infections, and glucocorticoid and tobacco use. Despite the overwhelming evidence of mortality in these patients, our patient responded to treatment. Thorough histories should be elicited from high-risk patients undergoing elective procedures prior to operation to address all possible complications. All patients should also undergo proper education including discussion of peri-operative and post-operative risks.
References
“
Johnston, Christine, and Anna Wald.



