-
PDF
- Split View
-
Views
-
Cite
Cite
Feyza Sönmez Topcu, Turgut İpek, Metin Kapan, Serpil Yılmaz, Fatih Ensaroğlu, Omental calcified fibrous tumor with symptoms after COVID-19 infection, Journal of Surgical Case Reports, Volume 2022, Issue 3, March 2022, rjac105, https://doi.org/10.1093/jscr/rjac105
Close - Share Icon Share
Abstract
Calcified fibrous tumor (CFT) is a rare benign tumor of mesenchymal origin. Between 1988 and 2019, a total of 272 CFT cases were reported. CFTs can be seen in all anatomical regions with soft tissue. Histologically, mononuclear inflammatory infiltrates and the presence of psammomatous calcification in dense hyalinized collagen are characteristic features of the tumor. Currently, if the tumor is located in only one focus, surgical removal is recommended. Although CFT is a benign tumor, it may cause complications. Diagnosis is often difficult due to the confusion of tumor findings with many diseases. We present a patient with CFT, whose omental lesions were detected on abdominal computed tomography, and the diagnosis was confirmed by histopathological examination.
INTRODUCTION
Calcified fibrous tumor (CFT) is a rare benign tumor of mesenchymal origin, which was first reported as a deep soft tissue tumor in children in 1988 and was accepted as an inflammatory or traumatic tumor [1, 2] in 2003, named as fibrous calcified tumor [3]. Between 1988 and 2019, a total of 272 CFT cases were reported [4]. CFTs can be seen in all anatomical regions with soft tissue [5, 6]. The etiology of the disease has not been adequately elucidated. Histologically, mononuclear inflammatory infiltrates and the presence of psammomatous calcification in dense hyalinized collagen are characteristic features of the tumor [6]. Currently, if the tumor is located in only one focus, surgical removal is recommended [6]. Although CFT is a benign tumor, it may cause complications [7]. Diagnosis is often difficult due to the confusion of tumor findings with many diseases. We present a patient with CFT, whose omental lesions were detected on abdominal computed tomography (CT), and the diagnosis was confirmed by histopathological examination.
CASE REPORT
A 28-year-old male patient was admitted to the hospital with complaints of abdominal pain, prolonged swelling after meals, diarrhea of three to four times a day, black stools and weight loss of 3 kg in the last 2 weeks. His complaints started about 2 weeks after treatment for the COVID-19 infection. Diagnostic colonoscopy was performed and a biopsy was obtained from the terminal ileum. Biopsy results were evaluated together with the clinical findings, leading to the preliminary diagnosis of Crohn’s disease, and three doses of budesonide 3 mg/dose were started. The patient did not benefit from 1 month of treatment. Control physical examination, acute phase reactants, liver and kidney function tests were normal. Abdominal CT was performed with intravenous and oral contrast material (300/50 ml iohexol). Tomography revealed multiple scattered millimeter-sized nodular calcifications superficially located in the omentum in the right and left lower quadrants, clustered nodular calcifications of ~3.5 × 2 and 3 × 1 cm and omental heterogeneity. Abdominal viscera were normal (Supplementary Images 1 and 2). Laparoscopy was performed, with diffuse omental calcific nodules, partial adhesion of the omental fat tissue to the small intestine at the pelvic level, and a small amount of free serous fluid in the pelvis was detected (Fig. 1). The tumoral structures were removed by resection of two omental fragments of 15 ×5 × 2 and 8 × 7 × 1.5 cm.
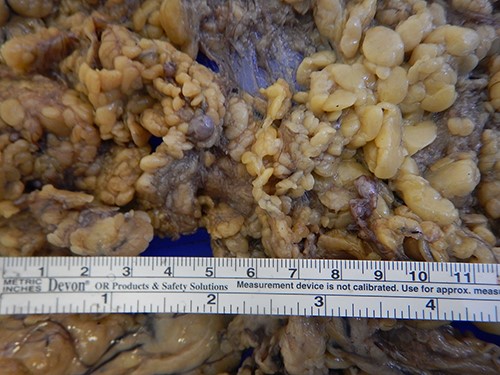
On macroscopic examination, numerous (>100) gray-white-colored grape-like hard nodules were seen, with the largest measuring 4 cm and the smallest measuring 0.2 cm. They were well-circumscribed, unencapsulated, spherical or lobulated and cut surfaces were homogenous, gray-white and firm to rubbery. Microscopically, hypocellular spindle cell proliferations embedded in abundant hyalinized collagen were seen, occasionally dystrophic or psammomatous calcifications interspersed with sparse lymphoplasmacytic infiltrate that may form lymphoid follicles. There was no mitosis, atypia or necrosis (Figs 2 and 3).
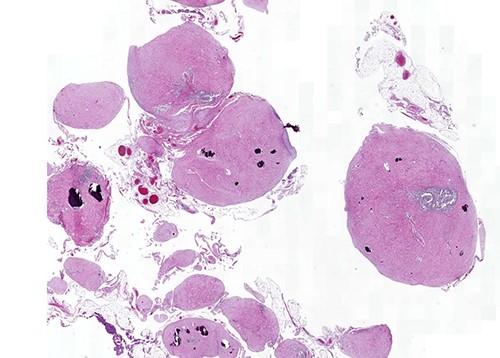
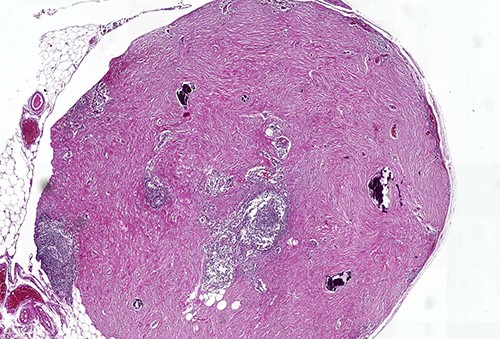
Calcifying fibrous tumor with abundant paucicellular hyalinized collagen, interspersed psammomatous or dystrophic calcifications and inflammatory infiltrate consisting of lymphocytes and plasmocytes (H&E, ×4).
In order to distinguish from other spindle cell tumors, immunohistochemical stains were performed. There was positive staining of the spindle cells for Factor XIIIa (Fig. 4) and a diagnosis of CFT was confirmed. Smooth muscle actin, beta-catenin, s-100, CD34, ALK, DOG1 and CD117 were negative; the IgG4/immunoglobulin G (IgG) ratio was 7.24.
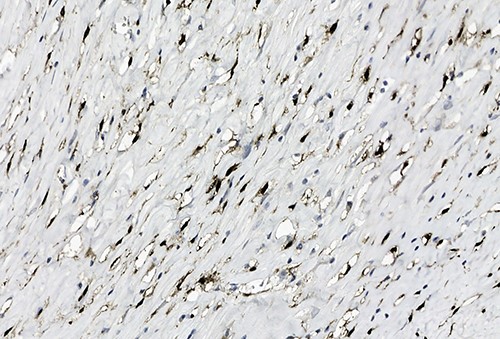
Positive staining of spindled tumor cells with Factor XIIIa, ×38.
After diagnosis, chest, neck and inguinal regions were scanned with CT to search for possible secondary foci, and no other foci were found. However, very small residual calcified foci were seen on the right and left omental surfaces of the abdomen. A laparotomy was performed to complete the tumor removal and no complications developed.
DISCUSSION
CFT is rare disease, its etiopathogenesis has not been fully elucidated yet, but it may be associated with trauma, previous surgical procedures and chronic infections [8]. It is more common in the pleura, abdominal cavity and neck, especially in the gastrointestinal tract. The median age of patients is 49 [9]. The disease is seen in both genders, slightly more common in women. Although CFTs are usually seen in a single focus, multifocal lesions have been defined in up to 10% of cases [10]. Most tumors are found incidentally, but large tumors may result in mass effect and local visceral symptoms. Gastrointestinal system symptoms involve mild abdominal pain or abdominal discomfort, anorexia, fever, weight loss, fatigue, dyspepsia, bloating, bad breath, nausea, vomiting, rectal bleeding, and changing bowel habits [11]. CT is better at detecting areas of early mineralization. Typically, CFT shows up as a homogeneous mass with well-defined borders, and mild enhancement and calcification on CT [11–12]. CFT is a benign tumor and local surgical resection is recommended for treatment.
CFTs are macroscopically well-circumscribed, unencapsulated, spherical or lobulated masses with various calcifications [13]. Microscopically, dense hyalinized stroma and hypocellular spindle cell proliferation are observed. There is no necrosis in the tumoral tissue. Scattered calcifications are common and can be psammomatous or dystrophic. Lymphoplasmacytic infiltrate is almost always present. CFTs should be differentiated from other spindle cell mesenchymal lesions, such as sclerosing calcified gastrointestinal stromal tumor, schwannoma, hyalinized leiomyoma, IgG4-related sclerosing disease, inflammatory myofibroblastic tumor, desmoid fibromatosis and solitary fibrous tumor. CFT tumor cells express Factor XIIIa, vimentin and CD34 but are insufficiently stained for CD117, DOG1 (Discovered on gastrointestinal stromal tumor 1), S100, actin, desmin, cytokeratin, ALK (Anaplastic lymphoma kinase), CD34 and beta-catenin [14]. In CFT, plasma cells can be stained positively for IgG and IgG4, increasing the likelihood that CFT is a manifestation of IgG4-related disease. However, increased IgG4 positive plasma cells is not specific and can be seen in various inflammatory conditions.
CFTs may be related to the late stage of inflammatory reactions due to infection, trauma or surgical procedures [15]. Our patient had no trauma or any other health problem other than the recent COVID-19 infection. COVID-19 and malignant diseases lead to overlapping inflammatory reactions at many points [16]. Although we do not have data to show the relationship between them, we think that studies on this subject are needed.
In conclusion, CFT is a tumor that should be kept in mind in the differential diagnosis of gastrointestinal system diseases, especially inflammatory bowel diseases. It can be treated with complete surgical resection.
CONFLICT OF INTEREST STATEMENT
None declared.



