-
PDF
- Split View
-
Views
-
Cite
Cite
Phillip J Whiley, Nicole Rodrigues, Janaka Balasooriya, A case of liver injury and pneumo-haemoperitoneum during pericardiocentesis, Journal of Surgical Case Reports, Volume 2022, Issue 2, February 2022, rjac009, https://doi.org/10.1093/jscr/rjac009
Close - Share Icon Share
Abstract
Pericardiocentesis is a generally safe procedure that provides effective resolution of cardiac tamponade. Emergency pericardiocentesis may be a life-saving intervention. Encountering an intra-abdominal organ in the path of the needle is predicted to be a potential complication in emergency subxiphoid approaches. Despite predictions of intraabdominal injuries, only few instances are recorded. In this case study, a patient recovering from percutaneous cardiac intervention required an emergency pericardiocentesis that was complicated by a liver injury, diaphragmatic penetration and pneumo-haemoperitoneum requiring surgical intervention to remove the drain. The case discusses options for performing the procedure, patient factors that can complicate the procedure and radiological and surgical diagnosis and treatment of this rare event.
INTRODUCTION
Pericardiocentesis is both a diagnostic and a potentially life-saving therapeutic procedure. Although considered relatively safe, this invasive procedure may be associated with certain risks and potentially serious complications. Major complications typically involve chamber lacerations, injury to vessels including coronary, intercostal, internal mammary or intraabdominal arteries or veins, pneumothoraces, arrythmia, pneumopericardium and bacteraemia [1–3].
When echocardiography guides the procedure for significant pericardial effusions, the success rate is >95% and the risk of complication is low. The morbidity rate is approximately 1–3% and the mortality rate from injuries directly caused by the procedure is less than 1% [3]. However, the need for urgent intervention at the bedside can complicate the procedure. Some mitigating strategies include echocardiography guidance systems with the needle mounted on the probe [4–6]. Also, studies show that over 95% of patients requiring pericardiocentesis after a cardiac intervention have received at least one anticoagulant or antiplatelet agent on the day of the procedure [7]. It is likely that the three agents prescribed for the patient in this case contributed to the significant haemoperitoneum.
Parasternal, apical and subxiphoid approaches are well described. The subxiphoid route is associated with the fewest complications [8]. The risks associated with the subxiphoid route, utilized in this case, include intraprocedural injury to the diaphragm, the phrenic nerve, inferior vena cava, hepatic vessels, liver, colon and stomach [9, 10]. In this approach, the needle is inserted between the xiphisternum and the left costal margin, directed towards the left shoulder at an angle of 30–45° angle to the skin with aspiration until pericardial fluid is obtained.
CASE REPORT
An 85-year-old male with a past medical history including unstable angina, hypertension and atrial fibrillation underwent elective percutaneous coronary intervention (PCI). There was no history of any abdominal or thoracic surgeries. His medications included apixaban, bisoprolol, ramipril, isosorbide mononitrate, aspirin, clopidogrel and digoxin. The procedure was uneventful. A right femoral artery approach was used, and a 6-French sheath was passed. A drug-eluting stent was placed to proximal first diagonal artery (D1) and proximal left anterior descending artery (LAD) across D1. A balloon was unable to be advanced through the LAD stent despite multiple attempts. The patient was admitted to the cardiac care unit for observation.
Several hours after the procedure the patient became acutely bradycardic (HR 38) and hypotensive (BP 56/46). Bedside echocardiography demonstrated a 1.5 cm pericardial effusion. Pericardiocentesis was performed via a subxiphoid approach under ultrasound guidance. Blood was drained from the pericardial tube. Two hours later the patient recorded a blood pressure of 78/49 mmHg requiring vasopressor support. The drain output was 100 ml. The haemoglobin level was 127 g/L pre-procedure and 110 g/L post-procedure. Repeat bedside echocardiography was performed and the patient was transferred for computerized tomography (CT) given an abdominal examination notable for epigastric pain (Fig. 1.)
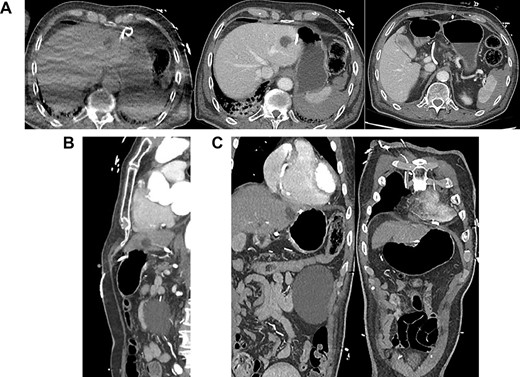
(A) Axial CT-angiogram: side drainage holes are apparent in the pericardium and the abdominal cavity; the drain courses anteriorly and abuts the medial aspect of the liver and stomach ~3 cm superiorly and medially to the course of the drain insertion forming a large loop; free gas is apparent at the right anterior liver; (B) sagittal CT-angiogram: subxiphoid approach with peritoneal entry; (C) coronal CT-angiogram: placement of catheter in pericardium; catheter traverses segment 2/3 liver.
The CT-angiogram showed no evidence of pericardial effusion, suggestive of successful treatment by drain placement (Fig. 1). It showed pneumo- and haemoperitoneum and that the drain appeared to course between the lesser curvature of the stomach and the liver with an intraperitoneal loop. There was no evidence of contrast leak to suggest active bleeding. A second CT of the abdomen with oral contrast provided no evidence of extravasation to suggest gastric perforation.
A diagnostic laparoscopy was undertaken to identify any intra-abdominal injuries and safe removal of drain. Laparoscopy findings confirmed an intraperitoneal course with a laceration in segment III of the liver and penetration of diaphragm (Fig. 2). There was clotted blood in the pelvis, which was likely due to liver injury. There was no evidence of viscus perforation identified to explain the pneumoperitoneum. More likely this air had entered during the initial procedure although a small viscus perforation that had already sealed would be a possibility. The pericardial drain was removed, and haemostasis confirmed. The patient was observed in the intensive care unit and made a full recovery.
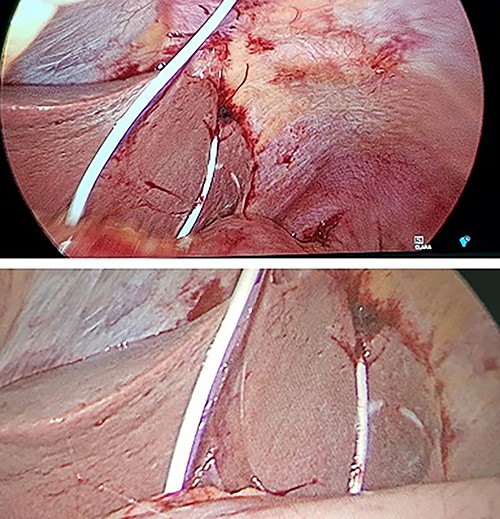
Intraoperative photographs during laparoscopy for removal of intraperitoneal pericardial drain.
DISCUSSION
The case presented here raises the risks associated with a subxiphoid approach and technical factors associated with emergency pericardiocentesis for post-PCI tamponade. The case also presents an optimal surgical remedy for misplaced intrabdominal pericardial tubes. The case highlights the need for CT imaging post-procedure for patients who experience abdominal pain and there is concern for an intraperitoneal injury. A significant amount of free gas in the abdomen is unusual with percutaneous procedures and CT scan with oral contrast does not completely rule out a viscus perforation. Performing laparoscopy examining for an intra-abdominal haemorrhagic site and achieving haemostasis, and to exclude any viscus injury are the key steps in managing pneumo-haemoperitoneum following pericardiocentesis.
These complications highlight the very real potential for adverse sequelae associated with pericardiocentesis and availability of expertise in the event of a major complication is invaluable.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
FUNDING
The authors received no funding for the study.



