-
PDF
- Split View
-
Views
-
Cite
Cite
Leva Gorji, James Augusta, Michael Elrod, Case report—successful management of acute buried bumper syndrome, Journal of Surgical Case Reports, Volume 2022, Issue 2, February 2022, rjac007, https://doi.org/10.1093/jscr/rjac007
Close - Share Icon Share
Abstract
Percutaneous gastrostomy (PEG) tube placement is often the preferred approach to addressing nutritional deficits in patients requiring long-term feeding access. Numerous major and minor complications may occur with PEG tube insertion; buried bumper syndrome is a rare, long-term outcome of PEG tube placement, comprising <2.4% of complications. We present the case of a 60-year-old female with laryngeal cancer whom developed acute buried bumper syndrome after PEG tube insertion which was managed successfully with surgical intervention.
INTRODUCTION
Percutaneous gastrostomy (PEG) tube placement is often the preferred approach to addressing nutritional deficits in patients requiring long-term feeding access. The benefits of PEG tube placement pertain to the continuous stimulation of the bowel. Complications with PEG tube placements occur in up to ~30% of patients, with the incidence of buried bumper syndrome (BBS) be ~0.3–2.4%.
CASE REPORT
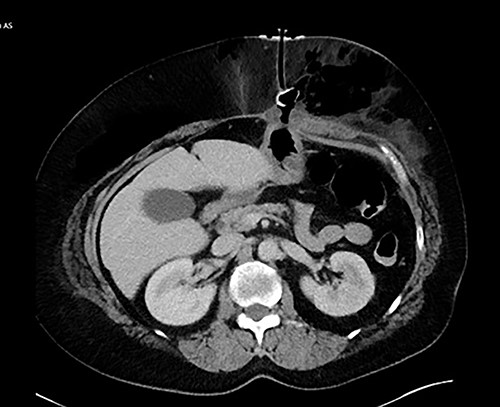
We present the case of a 60-year-old female who presented with a 3-month history of hoarseness and dysphagia in the setting of a left pyriform sinus hypopharyngeal lesion. The patient underwent evaluation in the operating room demonstrating deviation of the left arytenoid and aryepiglottic fold with associated enlargement and edema, prompting a tracheostomy placement in addition to lesion biopsy in order to secure a safe airway. The left pyriform sinus biopsy was remarkable for invasive, moderately differentiate squamous cell carcinoma. General surgery was consulted for placement of PEG tube secondary to high risks of aspiration and dysphagia caused by mass effect from the laryngeal cancer. A 20 French Corpak PEG tube was placed and secured with the internal retention bumper position appropriately against the gastric wall without tension; 2–0 prolene suture was utilized in order secure the bumper to the skin. Postoperative tube feeds were tolerated well and the patient was subsequently discharged from the hospital. The patient returned to the emergency department on postoperative Day 4 with complaints of significant, new onset abdominal pain. A computed tomography of abdomen and pelvis was obtained with evidence of BBS (Fig. 1). The patient was later taken to the operating room, where a laparoscopic gastrectomy was performed encompassing the necrotic portion of the stomach. The abdominal wall had developed an abscess in the left upper quadrant, prompting a laparoscopic 18F jejunostomy tube placement in order to avoid the previously involved region. The jejunum was then tacked to the abdominal wall and marked with 5 mm titanium endoclips in order to make replacement jejunostomy feasible with interventional radiology should the feeding access be dislodged. The abdominal wall abscess cavity measuring 30 × 20 × 10cm was appropriately debrided, the fascia approximated, and the wound closed with a negative pressure wound therapy (NPWT) device. The patient did inadvertently remove jejunostomy tube a few days later, which was replaced by interventional radiology. The patient tolerated tube feeds well and did not experience any other complications post operatively relating to feeding access. The NPWT sponge changes continued for ~2 months at which point the wound was amenable to simple packing and continued to heal appropriately.
DISCUSSION
PEG tube complications includes procedure-related complications as well as postprocedure complications. Procedure-related complications include risk of aspiration, bleeding, perforation of viscera with peritonitis and prolonged ileus. Risk factors of the procedure which contribute to aspiration include the supine position, sedation, neurological impairment and age; the risk of aspiration can be reduced with avoidance of over sedation, avoidance of excessive insufflation of the stomach and thorough aspiration of gastric contents [1, 2]. Acute bleeding is an uncommon complication, with risk factors being bleeding disorders and therapeutic anticoagulation [3]. Transient pneumoperitoneum occurs in ~50% of patients subsequent to PEG tube placement; however, this tends to be generally not be clinically significant [4]. Pneumoperitoneum becomes concerning when associated with signs of peritonitis, leukocytosis and evidence of visceral damage or leakage. Prolonged ileus is a rare complications occurring in 1–2% of patients, which typically is managed with conservative measures, including uncapping the PEG tube [5–7].
Postprocedure complication include PEG tube site infections, leakage and irritation surrounding PEG site, gastric ulcers or hemorrhage, fistulous tracts, inadvertent removal and BBS. PEG tubes have a high infection rate of ~30%, the vast majority are minor with <2% requiring aggressive medical or surgical intervention [8]. Risks factors for infection include poorly controlled diabetes, poor nutritional status, chronic steroid therapy and inadequate skin incision size preventing drainage of bacteria and gastric contents. Several studies have demonstrated the benefits of prophylactic broad-spectrum antibiotics 30 min prior to skin incision for prevention of site infections [9–12].
BBS, a result of gastric tube migration, is a rare complication occurring in up to 2.4% of the population; typically, presentation involves peritubal infection or leakage, abdominal pain, and resistance to formula infusion. Risk factors for developing this complication include malnutrition particularly immunocompromised patients with a body mass index <20, disorders or drugs associated with poor wound healing, and most importantly excessive tension between the internal and external bolsters—either through surgical technique or inadvertent pulling on the tube causing tension or dislodgement of the tube [9, 11, 12].
Only a limited number of published reports in English remark on the occurrence of BBS within the first month of PEG tube placement, with the earliest report occurring 3 days postprocedure in an Alzheimer’s patient [11, 13]. In this patient, the tube was removed by external traction under endoscopic visualization without resistance or signs of complication to the abdominal wall. The patient was admitted for antibiotics and observation, and no additional feeding access was pursued as patient was tolerating some level of oral intake. Follow-up imaging prior to discharge did not indicate evidence of complication [13].
The most commonly utilized classification of BBS is described by Orsi et al.
Notably, there are currently no formal guidelines describing the management of BBS. The treatment considerations described above [Table 1] [11] are based on typical experience of management reported in the literature, with the most widely described approach to management being endoscopic or surgical removal of the tube. Our case of BBS, classified as a grade III by Orsi et al. system, indeed, required surgical intervention with debridement, NPWT, and alternative feeding access.
BBS grading system described by Orsi et al. with the recommended treatments [11]
| Grade . | Description . | Treatment consideration . |
|---|---|---|
| I | Partial migration with asymptomatic presentation or mild symptoms such as abdominal pain or ostomy infection | Endoscopic management |
| II | Subtotal migration, in which the patient presents with dysfunction of the tube and extravasation of the feeding content | Surgical management |
| III | Total migration that is manifested by tube obstruction | Surgical management |
| Grade . | Description . | Treatment consideration . |
|---|---|---|
| I | Partial migration with asymptomatic presentation or mild symptoms such as abdominal pain or ostomy infection | Endoscopic management |
| II | Subtotal migration, in which the patient presents with dysfunction of the tube and extravasation of the feeding content | Surgical management |
| III | Total migration that is manifested by tube obstruction | Surgical management |
BBS grading system described by Orsi et al. with the recommended treatments [11]
| Grade . | Description . | Treatment consideration . |
|---|---|---|
| I | Partial migration with asymptomatic presentation or mild symptoms such as abdominal pain or ostomy infection | Endoscopic management |
| II | Subtotal migration, in which the patient presents with dysfunction of the tube and extravasation of the feeding content | Surgical management |
| III | Total migration that is manifested by tube obstruction | Surgical management |
| Grade . | Description . | Treatment consideration . |
|---|---|---|
| I | Partial migration with asymptomatic presentation or mild symptoms such as abdominal pain or ostomy infection | Endoscopic management |
| II | Subtotal migration, in which the patient presents with dysfunction of the tube and extravasation of the feeding content | Surgical management |
| III | Total migration that is manifested by tube obstruction | Surgical management |
CONCLUSION
In conclusion, we recommend the consideration of BBS, a traditionally late complication of PEG tube placement, as a potential early complication, as well. Based on our analysis and review of the literature, our case of BBS on postprocedure Day 4 is the earliest case reported in the literature which required surgical intervention.
CONFLICT OF INTEREST STATEMENT
None declared.
INFORMED CONSENT
All attempts have been exhausted in trying to contact the patient, next of kin, and/or parent/guardian for informed consent to publish their information, but consent could not be obtained.
REFERENCES
Parrish C, Lynch CR, Fang JC.



