-
PDF
- Split View
-
Views
-
Cite
Cite
Muhamad Zakaria Brimo Alsaman, Ahmad Anbar, Ahmad Nawlo, Adeeb Almooay, Ahmad Darwish, Mazen Mohammad, Incidental diagnosis of pseudomyxoma peritonei by laparoscopy: a rare case from Syria, Journal of Surgical Case Reports, Volume 2022, Issue 12, December 2022, rjac558, https://doi.org/10.1093/jscr/rjac558
Close - Share Icon Share
Abstract
Pseudomyxoma peritonei (PMP) is a rare malignancy of the abdomen, which is usually described as a gelatinous peritoneal fluid. A 33-year-old man came to the ER complaining of recurring abdominal pain with abdominal distention for the past 3 months. Abdominal ultrasound revealed moderate amount of turbulent ascitic fluid with septations, in addition to a mass with irregular margins consisting of liquid and cysts. Abdomen and pelvis computed tomography scan showed free abdominal fluid. A decision was made for abdominal diagnostic laparoscopy with biopsies from the peritoneum, mesenteries and the gelatinous fluid. We could not investigate all the abdominal cavities and appendix due to the presence of severe adhesions and tuberculosis suspension. The pathology report indicated PMP. The patient was referred to an oncologist for chemotherapy consultation. The diagnosis can be challenging, as the symptoms and signs vary from patient to another; most cases may be asymptomatic and discovered incidentally during laparoscopy.
INTRODUCTION
Pseudomyxoma peritonei (PMP) is exceedingly rare type of abdominal mucinous neoplasia, which was first reported by Rokitansky in 1842 as the gelatinous degeneration in the peritoneum originated from ovaries [1].
The incidence of PMP is estimated to be one per million individuals per year, which is reported more in female patients with a high rate of incidence with a median age of 54 years [2].
PMP mainly originates from appendix and ovary, and it rarely arises in large bowl, urachus, gastric teratoma and schwannoma [1–3].
Patients with PMP are diagnosed with non-specific symptoms, such as abdominal pain, increased abdominal ascites, hernia, ovarian mass and suspicion of appendicitis, or peritonitis. The vast majority of PMP cases are diagnosed incidentally at laparotomy or laparoscopy [4]. Here we report a rare case of PMP in a 33-year-old man.
CASE REPORT
A 33-year-old man came to the ER complaining of recurring periumbilical abdominal pain radiating to both flanks with abdominal distention for the past 3 months; there was weight loss despite normal appetite. On physical examination, there was ascites with a palpable mass in the epigastrium.
His lab workout showed negative Hepatitis panel, ESR was 44 and CRP was 47.
Abdominal ultrasound revealed intra-peritoneal turbulent flow with loculated fluid collection, in addition to a mass with irregular margins consisting of liquid and cysts, extending from the left upper quadrant to the suprapubic area and the right upper quadrant, which may indicate a congested or edematous mesentery.
Computed tomography scan of the abdomen and pelvis showed massive amount of free abdominal fluid and irregular margins of the liver (Fig. 1).
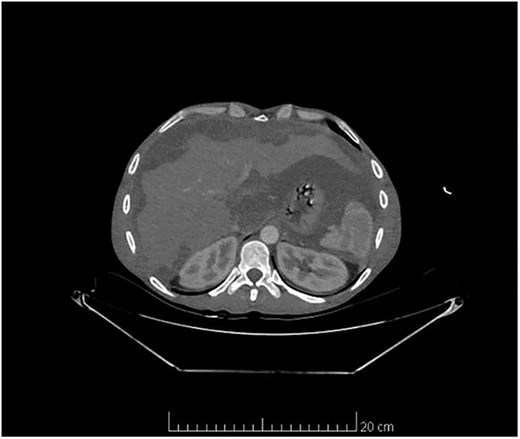
Computed tomography scan of the abdomen and pelvis showed massive amount of free abdominal fluid and irregular margins of the liver.
Paracentesis revealed gelatinous fluid with low albumin gradient ascites.
The patient was referred to general surgery department and a decision was made for abdominal diagnostic laparoscopy.
Laparoscopy showed severe adhesions and the abdomen cavity was full of mucus gelatinous fluid (Fig. 2). The rest of the abdominal cavities and appendix were not investigated due to the presence of severe adhesions and tuberculosis suspension.
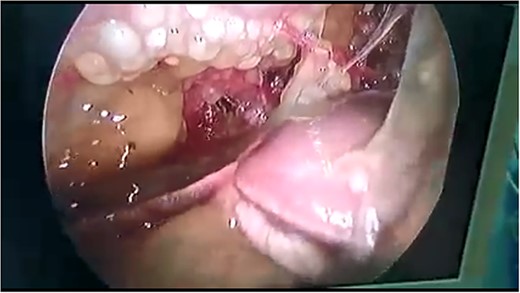
Abdominal laparoscopy showed a huge amount of mucus gelatinous fluid.
Biopsy samples were obtained from peritoneum, omentum and the gelatinous fluid.
The pathology report prescribed pools of mucus without malignant cells, which is indicative of PMP (Fig. 3).
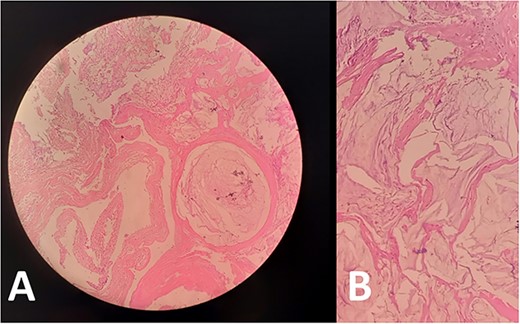
Biopsies from (A) peritoneum and (B) omentum showed acellular mucin consistent with PMP. No mucinous, degenerative or tumor cells are seen.
Ascites fluid is negative for malignant cells. Upper and lower gastric endoscopy was performed to rule out neoplasms. Upper endoscopy revealed gastritis in stomach and colonoscopy showed single sessile polyp measuring 0.5 cm in diameter located 20 cm from the anus. The patient was referred to an oncologist for chemotherapy consultation.
DISCUSSION
PMP was first described in the medical literature in 1842 by Rokitansky as gelatinous degeneration in the peritoneum [1]. It is considered as a very rare disease, reported more in female patients with a high rate of incidence.
The main origin is most commonly believed to be from the appendix and the ovary. For women, the origin of the ovary may be due to metastases from the appendix; Therefore, appendectomy has become a routine procedure during laparoscopic staging of ovarian tumors in many institutions [5–7].
We could not investigate the rest of the abdominal cavities and appendix due to the presence of severe adhesions and tuberculosis suspension.
But it may arise from any organ in the abdomen, colon, intestine and even malignant schwannoma. This disease rarely spreads through the lymphatic system or through the bloodstream, it is characterised by mucin and cancerous cells in the abdominal cavity. Its spread and distribution in the cavity of the peritoneum follows a distribution subject to physical factors such as the nature of mucous and gravity. Douglas’ diverticulum, rectosigmoid region, and pyloric region are common, but any abdominal organ possible. Diagnosing the condition can be challenging, as the symptoms and signs vary greatly from one patient to another; most cases may be asymptomatic and discovered incidentally during laparoscopy for other reasons. But it may be present with abdominal symptoms, nausea, vomiting, anorexia, weight loss and other non-specific symptoms, as in our case [4, 8].
The gold standard in PMP diagnosis is CT with contrast, and the diagnosis with ultrasound is difficult, but it may show high echogenic fluid and a lot of septations in the abdominal cavity [4, 9].
Because it is an uncommon disease, there is no standard in management, so some studies recommend watchful waiting and another recommend cytoreductive surgery with hyperthermic intra-peritoneal chemotherapy (HIPEC) or early post-operative intra-peritoneal chemotherapy [10, 11].
Nowadays complete macroscopic tumor excision termed cytoreductive surgery (CRS) combined with HIPEC is considered as ideal treatment.
The total operative time of this procedure is 10 h. It is performed by bilateral parietal and diaphragmatic peritonectomies, right hemicolectomy, radical greater omentectomy with splenectomy, cholecystectomy and liver capsulectomy, a pelvic peritonectomy with, or without, rectosigmoid resection and bilateral salpingo-oophorectomy with hysterectomy in females [12].
In general, intra- and post-operative chemotherapy improves the survival [13].
A systematic review and meta-analysis showed that combining cytoreductive surgery and intra-peritoneal chemotherapy improves the prognosis, although there is an increase in the morbidity rate [14]. Management such difficult cases require qualified centers and specific staffing requirements.
CONCLUSION
PMP is exceedingly rare type of abdominal mucinous neoplasia. The diagnosis can be challenging, as the symptoms and signs vary from patient to another; most cases may be asymptomatic and discovered incidentally during laparotomy or laparoscopy for other reasons. CRS combined with HIPEC is considered as the ideal treatment.
DATA AVAILABILITY
All data are included in this published article and its supplementary information files.
ACKNOWLEDGEMENTS
We would like to thank Dr. Mohamad Osama Hambrosh, the Oncologist for his great support in this case.
CONFLICT OF INTEREST STATEMENT
No conflict of interest.
FUNDING
There are no sources of funding.
DECLARATION OF PATIENT CONSENT
The authors declare that they have obtained the parents’ consent.
AUTHORS’ CONTRIBUTIONS
M.Z.B.A., A.A., A.N.: analyzed and interpreted the patient data, wrote the manuscript and revision.
A.D.: interpretation and providing pathological data.
A.M.: performed the laparoscopy.
M.M.: supervision, critical review and revision.
All authors read and approved the final manuscript.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- consultation
- abdominal pain
- computed tomography
- biopsy
- cancer
- chemotherapy regimen
- adhesions
- peritoneal fluid
- cysts
- laparoscopy
- mesentery
- pseudomyxoma peritonei
- suspensions
- syria
- tuberculosis
- abdomen
- diagnosis
- pathology
- pelvis
- peritoneum
- abdominal swelling
- abdominal ultrasonography
- Abdominal cavity
- oncologists



