-
PDF
- Split View
-
Views
-
Cite
Cite
Ioannis Zachos, Lampros Mitrakas, Foteini Karasavvidou, Anastasios Karatzas, Vassilios Tzortzis, Clear-cell renal cell carcinoma single urinary bladder metastasis: a case report and review of the literature, Journal of Surgical Case Reports, Volume 2022, Issue 10, October 2022, rjac475, https://doi.org/10.1093/jscr/rjac475
Close - Share Icon Share
Abstract
Renal cell carcinoma (RCC) is the most frequent solid lesion accounting for ~90% of all kidney malignancies. Clear-cell RCC (ccRCC) represents the most frequent subtype. Urinary bladder is a rare metastatic site either synchronous or metachronous. Hereby, we report the case of an 85-year-old male patient with a single urinary bladder metastasis due to ccRCC and we present a review of the literature.
INTRODUCTION
Kidney cancer is the 14th most common malignancy in humans and represents the third most common tumor of the urogenital system [1]. Annually, 431.288 new cases and 179.368 deaths are documented worldwide [1]. Renal cell carcinoma (RCC) is the most frequent solid lesion accounting for ~90% of all kidney malignancies. Clear-cell RCC (ccRCC) represents the most frequent subtype and it is associated with loss of chromosome 3p and mutation of the von Hippel–Lindau (VHL) gene at chromosome 3p25. Lung, lymph nodes, bone and liver are the usual metastatic sites [2]. Urinary bladder is a rare metastatic site either synchronous (23%) or metachronous (77%; [3]). Less than 70 cases are reported so far. Hereby, we present the case of an 85-year-old male patient with a single urinary bladder metastasis due to ccRCC.
CASE REPORT
An 85-year-old Caucasian male patient presented in the emergency department of our hospital with macroscopic hematuria. Anamnestically, he had a radical nephrectomy on the left side because of ccRCC before 12 months and a history of multiple thoracic metastases, which were found on the follow-up 6 months after the nephrectomy. Regarding this metastatic burden, the patient was treated with immunotherapy. Urinary bladder ultrasound and abdomen computer tomography showed a papillary mass on the right ureteric orifice (30 mm). A transurethral bladder resection of the tumor (TURBT) was performed ~2 weeks later. Histopathological examination revealed a non-muscle-invasive clear-cell carcinoma of the urinary bladder with the same characteristics as the primary kidney tumor. The immunohistochemical exam was positive for AE1/AE3, cyt7, GATTA3, p63, PAX8, vimentine and EMA keratins antibodies (Fig. 1), whereas it was negative for cyt20 and CD10. The patient is alive 16 months after the radical nephrectomy. The first follow-up cystoscopy at 3 months after TURBT showed no sign of bladder relapse. The patient undergoes cystoscopy and urine cytology examination every 3 months and he continues to be treated with immunotherapy by the oncologists of our institution.
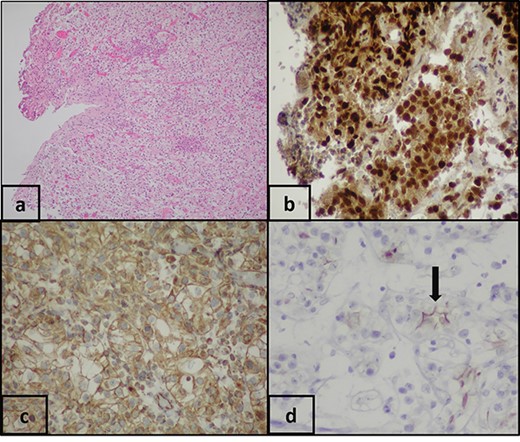
(a) Pathologic examination shows bladder biopsy with clear-cell carcinoma (H&E, ×100). Immunohistochemical staining shows that the clear cells are positive for: (b) PAX-8 (PAX-8, ×200), (c) vimentin (vimentin, ×200) and (d) EMA (EMA, ×200).
DISCUSSION
Frequent sites of RCC metastases, in priority order, are: (i) regional lymphnodes, (ii) lung, (iii) liver, (iv) bones, (v) adrenal glands, (vi) brain and (vii) skin. Furthermore, stomach adenocarcinoma, melanoma, breast cancer and colon adenocarcinoma, are the tumors that metastasize more often in the bladder. Urinary bladder metastases due to RCC rarely happen [4] and represent <2% of all bladder tumors. At diagnosis, median size is 21 mm and ~67% of them are non-muscle invasive [3]. Patients with RCC bladder metastases present themselves usually with macroscopic hematuria. It is interesting that 30% of RCC patients experience hematuria, whereas 68% of patients with metachronous bladder metastasis have this symptom at the diagnosis of the primary renal mass without evidence for bladder tumor, which suggests a breach of the pelvicalyceal system and that the bladder metastasis is due to spreading of malignant cells through the urinary apparatus [3]. Owing to the fact that a tumor could be located in the bladder neck, urinary retention is a less common manifestation. Notably, we have to mention that the patients delineate, at presentation, a history of renal cancer.
There is a controversy regarding the metastatic mechanism and the suggested routes are: (i) hematogenous: dissemination through systemic circulation [3] or through the gonadal vein to the bladder arising from the venous embolism of cancer cells or tumor invasion into the left renal vein [5, 6], (ii) lymphatic: interconnections between lymphatic and vascular channels allow retrograde tumor dissemination by the lymphatic system [7] and (iii) via the urinary system: cancer cells are detected in the urine of patients suffering from RCC [8].
The treatment of these secondary lesions is usually the TURBT. This is reasonable because selective metastasectomy has a role in metastatic RCC despite the poor final outcome [3]. The choice of radical cystectomy remains but it seems to be rational only for the lesions that will be proved to be pathologically muscle-invasive. Moreover, systemic therapy is indispensable if there are concomitant metastases in other organs. To our knowledge, <70 cases of ccRCC vesical metastases are found in the literature and the reported frequency rates range between 0.3 and 1.6%. The documented median time between nephrectomy and bladder metastasis is ~33 months [9]. The 2-year cancer-specific survival (CSS) rate in cases with a solitary bladder metastasis is 71.1% and in patients with more distant metastases is 25.8% [3]. At present, a safe estimation about the prognosis of the patients is not easy to be made, because of the limited number of the cases. The interval between diagnoses of RCC and bladder metastasis seems to have an impact on prognosis. Specifically, patients with bladder metastasis in the first 12 months after nephrectomy have a worse CSS rate in comparison to those who are affected after the abovementioned period (2-year CSS rates: 34.6 versus 58.4%, P = 0.063) [3].
CONCLUSION
RCC metastasizes in the urinary bladder rarely. Regarding treatment, there are no specific guidelines for this metastatic entity. Currently, the treatment is based on TURBT or cystectomy in case of lesions solely affecting the bladder. Finally, such patients have a relative short survival and those who are diagnosed with bladder metastasis within 1 year after the initial diagnosis of RCC have a poorer prognosis.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
All authors declare that there is no financial support for this work.



