-
PDF
- Split View
-
Views
-
Cite
Cite
Chai Wei Tong, Ashish Jiwane, A complicated case of terminal ileitis post-COVID-19 infection requiring bowel resection, Journal of Surgical Case Reports, Volume 2022, Issue 10, October 2022, rjac457, https://doi.org/10.1093/jscr/rjac457
Close - Share Icon Share
Abstract
Since the beginning of the COVID-19 pandemic, there have been reports of children developing systemic hyperinflammatory response to COVID-19 infection, known as Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-COV-2 (PIMS-TS). Here we would like to discuss a case of a 15-year-old male with PIMS-TS presenting as complicated terminal ileitis, requiring ileocaecal resection. Histopathologic findings of the ileocaecal specimen revealed thickened bowel mucosa, with features of granulomatous inflammation similar to Crohn’s disease, without features of intestinal vasculitis or viral particles. His post-operative course was complicated by fresh rectal bleeding, requiring urgent blood transfusion and endoscopy. Source of bleeding was not identified and his bleeding self-resolved. Gut biopsies of the remaining bowel taken during endoscopy were normal. Although this case suggests that the inflammatory process is focal to the terminal ileum with a good short-term prognosis, further follow-up is required to assess for long-term effects and risks of bowel disease.
INTRODUCTION
Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-COV-2 (PIMS-TS) refers to a systemic hyperinflammatory response to COVID-19 infection. PIMS-TS is also often referred to as Multisystem Inflammatory Syndrome in Children. Presentation of PIMS-TS includes current or recent COVID-19 infection, persistent fever, elevated inflammatory markers and single or multi-organ dysfunction, in the absence of other microbial infections [1]. Common gastrointestinal manifestations of the disease include acute abdominal pain, vomiting and diarrhoea. Most reported cases so far recover with medical therapy, rarely surgical resection is required [2]. Here we would like to discuss a case of a 15-year-old male with PIMS-TS, who presented with complicated terminal ileitis 8 weeks post-COVID-19 infection, requiring ileocaecal resection.
CASE REPORT
H is a previously well 15-year-old male who presented to the paediatric emergency department with an acute worsening right-sided abdominal pain, fevers and vomiting, 4 weeks post-discharge from hospital with COVID-19-related encephalopathy. COVID-19 was diagnosed 8 weeks prior to this presentation.There is no family history of bowel disease. On examination, he had a soft, non-distended abdomen but had rebound tenderness in the right iliac fossa. The pain was not relieved by simple analgaesia. His vital signs recorded a respiratory rate of 18 brpm, oxygen saturation of 98% in room air, heart rate of 112 bpm, blood pressure 108/67 mmHg and a temperature of 39°C.
Initial blood investigations showed an elevated C-reactive protein (100), neutrophilia (8.51 × 109/L), raised ferritin (118ug/L), raised D-dimer (1.68 mg/L) and lymphopenia (1.17 × 109/L). His blood cultures were negative. Abdominal ultrasound found a thickened distal ileum with surrounding inflammatory phlegmon and possible microperforation. The appendix, however, appeared normal. Due to the unusual findings of the ultrasound and abnormal labaratory tests, he underwent an abdominal computed tomography (CT), which reported a long segment circumferential thickening of bowel wall involving ileocaecal valve to distal and terminal ileum. There was an associated area of phlegmon adjacent to the mesentery measuring 29 × 32mm (Fig. 1). The overall investigations met the diagnostic features for PIMS-TS as per guidelines from the Royal College of Paediatrics and Child Health (Table 1) [1].
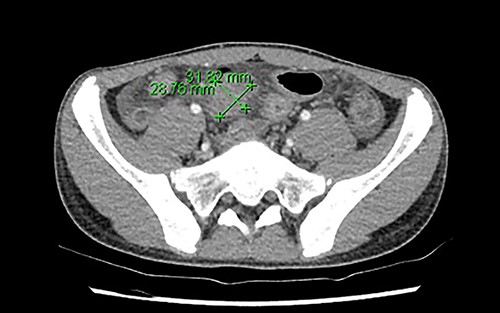
The region of phlegmon in the adjacent ileal mesentery, measuring about 29 × 32 mm.
| Clinical . | Laboratory . | Imaging . |
|---|---|---|
| • All | • All: | • Echo |
| • Persistent fever >38.5°C | • Abnormal fibrinogen | • Myocarditis, pericardial effusion |
| • Most: | • Absence of other infectious cause | • CXR |
| • Oxygen requirement | • ↑ C reactive protein | • Patchy symmetrical infiltrates, pleural effusion |
| • Hypotension | • ↑ D-Dimers | • US abdomen |
| • Some: | • ↑ ferritin | • Colitis, ileitis, lymphadenopathy, ascites, |
| • Gastrointestinal (abdominal pain, diarrhoea, vomiting) | • ↑Neutrophils | hepatosplenomegaly |
| • Neurological (confusion, headache) | • ↓ Albumin | |
| • Skin (rash) | • ↓Lymphocytes | |
| • Respiratory (sore throat) | • Some | |
| • Acute kidney injury | ||
| • Anaemia | ||
| • Coagulopathy | ||
| • Proteinuria | ||
| • ↑ CK | ||
| • ↑ LDH | ||
| • ↑ triglycerides | ||
| • ↑ troponin | ||
| • Thrombocytopenia | ||
| • Transaminitis |
| Clinical . | Laboratory . | Imaging . |
|---|---|---|
| • All | • All: | • Echo |
| • Persistent fever >38.5°C | • Abnormal fibrinogen | • Myocarditis, pericardial effusion |
| • Most: | • Absence of other infectious cause | • CXR |
| • Oxygen requirement | • ↑ C reactive protein | • Patchy symmetrical infiltrates, pleural effusion |
| • Hypotension | • ↑ D-Dimers | • US abdomen |
| • Some: | • ↑ ferritin | • Colitis, ileitis, lymphadenopathy, ascites, |
| • Gastrointestinal (abdominal pain, diarrhoea, vomiting) | • ↑Neutrophils | hepatosplenomegaly |
| • Neurological (confusion, headache) | • ↓ Albumin | |
| • Skin (rash) | • ↓Lymphocytes | |
| • Respiratory (sore throat) | • Some | |
| • Acute kidney injury | ||
| • Anaemia | ||
| • Coagulopathy | ||
| • Proteinuria | ||
| • ↑ CK | ||
| • ↑ LDH | ||
| • ↑ triglycerides | ||
| • ↑ troponin | ||
| • Thrombocytopenia | ||
| • Transaminitis |
Note: Adapted from Royal College of Paediatrics and Child Health [1].
| Clinical . | Laboratory . | Imaging . |
|---|---|---|
| • All | • All: | • Echo |
| • Persistent fever >38.5°C | • Abnormal fibrinogen | • Myocarditis, pericardial effusion |
| • Most: | • Absence of other infectious cause | • CXR |
| • Oxygen requirement | • ↑ C reactive protein | • Patchy symmetrical infiltrates, pleural effusion |
| • Hypotension | • ↑ D-Dimers | • US abdomen |
| • Some: | • ↑ ferritin | • Colitis, ileitis, lymphadenopathy, ascites, |
| • Gastrointestinal (abdominal pain, diarrhoea, vomiting) | • ↑Neutrophils | hepatosplenomegaly |
| • Neurological (confusion, headache) | • ↓ Albumin | |
| • Skin (rash) | • ↓Lymphocytes | |
| • Respiratory (sore throat) | • Some | |
| • Acute kidney injury | ||
| • Anaemia | ||
| • Coagulopathy | ||
| • Proteinuria | ||
| • ↑ CK | ||
| • ↑ LDH | ||
| • ↑ triglycerides | ||
| • ↑ troponin | ||
| • Thrombocytopenia | ||
| • Transaminitis |
| Clinical . | Laboratory . | Imaging . |
|---|---|---|
| • All | • All: | • Echo |
| • Persistent fever >38.5°C | • Abnormal fibrinogen | • Myocarditis, pericardial effusion |
| • Most: | • Absence of other infectious cause | • CXR |
| • Oxygen requirement | • ↑ C reactive protein | • Patchy symmetrical infiltrates, pleural effusion |
| • Hypotension | • ↑ D-Dimers | • US abdomen |
| • Some: | • ↑ ferritin | • Colitis, ileitis, lymphadenopathy, ascites, |
| • Gastrointestinal (abdominal pain, diarrhoea, vomiting) | • ↑Neutrophils | hepatosplenomegaly |
| • Neurological (confusion, headache) | • ↓ Albumin | |
| • Skin (rash) | • ↓Lymphocytes | |
| • Respiratory (sore throat) | • Some | |
| • Acute kidney injury | ||
| • Anaemia | ||
| • Coagulopathy | ||
| • Proteinuria | ||
| • ↑ CK | ||
| • ↑ LDH | ||
| • ↑ triglycerides | ||
| • ↑ troponin | ||
| • Thrombocytopenia | ||
| • Transaminitis |
Note: Adapted from Royal College of Paediatrics and Child Health [1].
A multi-disciplinary team comprising of surgical, immunology and gastroenterology teams were involved in his care. He was commenced on conservative management with intravenous antibiotics, intravenous immunoglobulin, nil by mouth and total parenteral nutrition for 2 weeks, followed by a slow upgrade in diet for the following 2 weeks. Unfortunately, his pain did not completely resolve, and progress Positron Emission Tomography-CT identified ongoing inflammation in the terminal ileum and further to the rectosigmoid junction (Fig. 2).
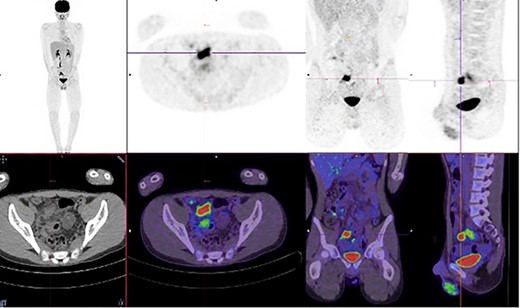
Increased metabolic activity in the terminal ileum and anterior to the rectosigmoid junction to the right of midline.
The decision to proceed with a diagnostic laparoscopy plus laparotomy was made following discussion with the patient and his family. Intraoperatively, a 15-cm long inflammatory mass was found adherent to the right lateral pelvic wall. This mass consisted of an inflamed thickened terminal ileum with fat wrapping, the appendix could not be identified and is suspected to be a part of the inflammatory mass. The sigmoid colon was adherent to this mass and was able to be gently separated from the ileum with no perforation identified. An ileocaecal resection was performed. The macroscopic appearance of the ileum revealed inflammation extending to within 5 cm of the ileocaecal wall with mesenteric lymphadenopathy, similar to features seen in Crohn’s ileitis (Figs 3 and 4). Formal histopathology of the specimen showed mixed features of granulomatous inflammation similar to Crohn’s disease as well as features similar to necrotising enterocolitis, there were no features of intestinal vasculitis.
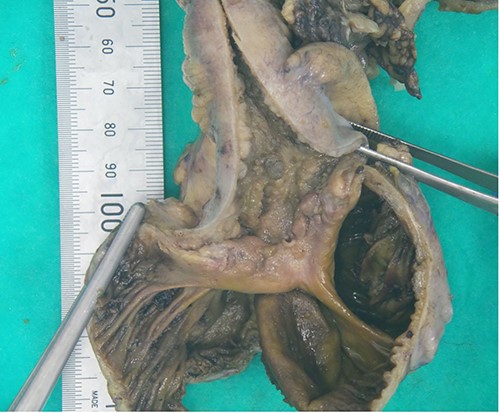
Cut open ileocaecal specimen showing thickened, oedematous and inflamed intestinal wall.
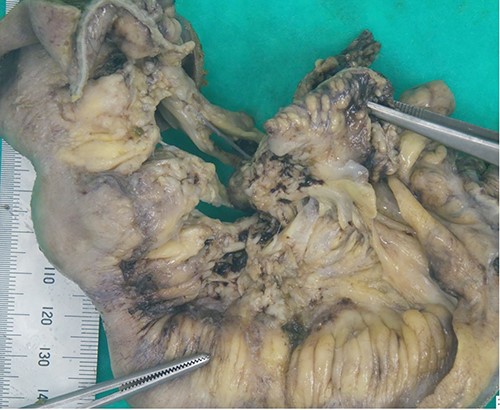
Ileocaecal resection specimen showing inflammatory phlegmon secondary to perforation arising from the terminal ileum.
His post-operative course was complicated by gastrointestinal bleeding (haemoglobin drop from 101 to 54 g/L), where he required an urgent endoscopic evaluation. The source of the bleeding was not able to be identified and his bleeding self-resolved. Biopsies were opportunistically taken during the endoscopy which revealed normal mucosa without features of inflammatory bowel disease (IBD). He was subsequently discharged home and has remained pain free with no further bleeding. An outpatient small bowel magnetic resonance enterography performed 4 weeks post-discharge was normal, without evidence of IBD or features of mechanical obstruction.
DISCUSSION
Terminal ileitis refers to an inflammation of the terminal ileum. Main symptoms of terminal ileitis are right lower quadrant abdominal pain and diarrhoea. Some patients may also present with bleeding, weight loss and possible bowel obstruction [3]. Ileitis is most often caused by Crohn’s disease; however, infectious diseases, vasculitis, ischaemia and spondyloarthropathies can be associated with ileitis.
PIMS-TS was first identified in April 2020 and shares features of both Kawasaki disease and toxic shock syndrome [4]. Diagnostic criteria include a persistent fever with single or multiorgan dysfunction, evidence of inflammation (neutrophilia, lymphopenia, raised C reactive protein), plus additional features, for example, abdominal pain, diarrhoea and vomiting (Table 1). Microbial causes need to be excluded before this diagnosis can be made [1].
Current treatment of PIMS-TS is often multidisciplinary and includes supportive care, intravenous immunoglobulin therapy, steroids and biological therapies [5, 6]. There has been a few reports of new IBD in adolescents after or concurrent COVID-19 infection [7, 8]. SARS-CoV-2 is known to bind to Angiotensin-converting enzyme-2 receptor on intestinal epithelial cells, causing overproduction of proinflammatory cytokines, promoting intestinal inflammation. It is speculated that an individual with dysregulated immune response from SARS-CoV-2 will develop an intense intestinal inflammation, triggering onset of IBD [9]. Although our patient in this case has not had any evidence of IBD to date, further monitoring and follow-up would be useful to assess whether these reports are pure coincidence versus a chronic progression of an initial acute hyperinflammatory illness. SECURE-IBD is a registry that stores de-identified cases of COVID-19 in populations with IBD, which may be of a helpful reference to study the links between COVID-19 and IBD [10].
CONCLUSION
With the rising numbers of COVID-19 infections, PIMS-TS should be considered as a major differential diagnosis in patients presenting with atypical acute abdominal pain. Multidisciplinary team involvement is recommended in the management of these cases and there is a potential role of surgical treatment. Further monitoring and research are required to assess the long-term effects and risks of bowel disease from PIMS-TS.
ACKNOWLEDGEMENTS
The author thank Dr Ashish Jiwane, Consultant Paediatric Surgeon at Sydney Children’s Hospital, Randwick, NSW, Australia for his support of this report as well as leading the management of this patient. The author also thank the Anatomical Pathology Department of Prince of Wales Hospital, Randwick, NSW, Australia for supplying photos of the cut-up surgical specimen.
CONFLICT OF INTEREST STATEMENT
The author does not have any conflicts of interest to declare.
References
- hemorrhage
- vasculitis
- bowel resection
- crohn's disease
- endoscopy
- blood transfusion
- child
- follow-up
- intestinal diseases
- intestines
- virion
- infections
- mucous membrane
- rectal bleeding
- distal ileum
- granulomatous inflammation
- crohn's disease of terminal ileum
- histopathology tests
- intestinal biopsy
- sars-cov-2
- covid-19
- coronavirus pandemic
- pediatric inflammatory multisystem syndrome



