-
PDF
- Split View
-
Views
-
Cite
Cite
Joseph Frantzias, Prajwal Ghimire, Jose P Lavrador, Noemia Pereira, Ranjeev Bhangoo, Nicholas Thomas, Combined extradural and intradural approach to a trigeminal nerve hemangiopericytoma with cranial nerve monitoring: a technical note of a rare case, Journal of Surgical Case Reports, Volume 2022, Issue 10, October 2022, rjac445, https://doi.org/10.1093/jscr/rjac445
Close - Share Icon Share
Abstract
Hemangiopericytoma (HPC) of the trigeminal nerve is extremely rare. We present a case of a large cystic HPC of the mandibular division of the trigeminal nerve, only the third case described in the literature, with both intradural and extradural components. We describe the surgical approach, assisted by neurophysiological techniques of mapping and monitoring including blink reflex and triggered electromyography. Additionally, we report a method of monitoring of the sensory branches of the trigeminal nerve, poorly described in the literature, through peripheral and direct nerve stimulation and recording of transcranial somatosensory evoked potentials.
INTRODUCTION
Hemangiopericytoma (HPC) of the meninges is a rare tumor, classified by WHO under the category of solitary fibrous tumor of the dura. First described in 1928 [1], most of them are locally invasive, and tend to grow along venous sinuses, which often requires adjuvant radiotherapy [2]. Recurrence as well as intra-cranial and extra-cranial metastases are a bad prognostic sign [3]. HPCs of the cranial nerves are extremely rare and literature revealed two reported cases of HPC of the trigeminal nerve [4]. We present a case of HPC of the mandibular division of trigeminal nerve and our surgical strategy for its resection.
CASE REPORT
A 32-year-old man presented with a 4-week history of worsening headaches, and a 1-week history of nausea and vomiting associated with memory problems over a period of months. Neurological examination was unremarkable. Computed tomography (CT) scan with contrast revealed a left temporal enhancing lesion with enlargement of the foramen ovale (Fig. 1a) and an magnetic resonance imaging (MRI) scan confirmed the presence of a large lesion (43 x 45 x 54 mm) lying in the temporal fossa, with mixed cystic and solid enhancing components (Fig. 1b). After discussion at the neuro-oncology multi-disciplinary meeting, a decision was made for surgical resection of the lesion.
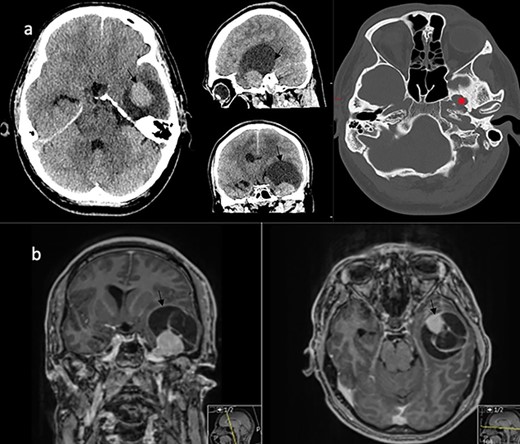
Imaging characteristics of the V3 lesion. (a) CT scan axial, sagittal and coronal views (brain and bone window) showing the lesion in the left middle fossa with enlargement of the foramen ovale (asterix). The lesion (arrow) is uniformly enhancing with a large capping cyst causing mass effect. (b) MRI scan coronal and axial views showing a mixed solid and cystic lesion (arrow) in proximity to the Meckel’s cave.
SURGICAL TECHNIQUE
The patient was positioned supine in a Mayfield clamp, with the head turned to the right by 45°. A skin incision was performed as outlined in Figure 2a, and a pterional craniotomy and interfascial approach to the temporalis was completed. The lesser and greater sphenoid wings were drilled (Fig. 2b). The meningo-orbital band was identified and incised (Fig. 2c).
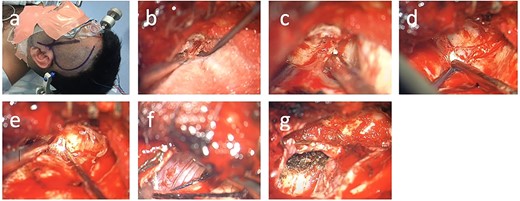
Surgical technique. (a) Skin incision; (b) drilling of the lesser wing of the left sphenoid bone; (c) incision of the meningo-orbital band, (d) dura propria and periosteal dura at the superior orbital fissure, (e) dura propria and periosteal dura at the foramen rotundum, (f) tumor coming into view at the foramen ovale, (g) visualization of the foramen ovale from intradurally after tumor debulking.
Both anterior and middle skull base were thus exposed. The cystic component was drained first via a dural stab incision with a navigation guided Dandy canula, draining 20 ml of serous fluid. This maneuver allowed decompression of the bulging temporal lobe and facilitated further exposure of the middle fossa. Following that, Kerrison rongeurs were used to enlarge the superior orbital fissure (Fig. 2d). Dissection then proceeded between the two layers of the dura to expose first the foramen rotundum, and finally the foramen ovale with the tumor coming into view inter- and extra-durally (Fig. 2f–g).
Mapping the margins using monopolar stimulation revealed no positive responses, and therefore we preceded to incise the tumor capsule and partially debulk the lesion. Frozen section suggested histology consistent with HPC. The foramen ovale was widened and the tumor was removed from that area as well. The integrity of the all divisions of the trigeminal nerve were continuously monitored throughout via somatosensory evoked potential (SSEP) recordings from scalp electrodes, and via direct nerve stimulation of the sensory and motor branches, and blink reflex monitoring (Figs 3–6). All monitoring modalities were replicable and stable throughout tumor removal. The dura over the temporal lobe was then opened. The tumor was identified and extensively debulked with a ultrasonic aspirator (SonopetR, Stryker, Michigan USA). The capsule was dissected from the left temporal lobe and the whole intradural component was removed. The foramen ovale and the trigeminal nerve were observed from the intradural space (Fig. 2g).
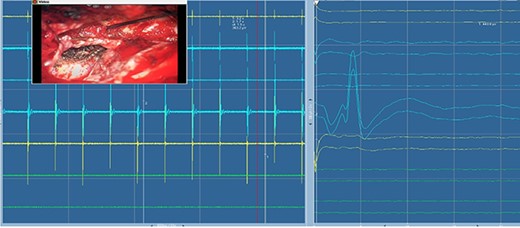
Electromyography of trigeminal nerve (motor). Trigger EMG of the motor branches of the trigeminal nerve with positive motor responses (mentalis muscle EMG). Nearby cranial nerves were additionally monitored including the facial nerve CN VII (orbicularis oculi, orbicularis oris and mentalis muscle) and abducens nerve CN IV (lateral rectus muscle).
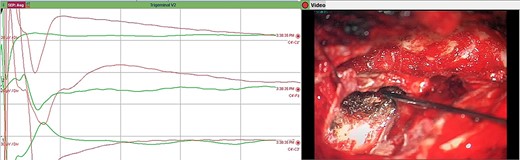
Direct nerve stimulation of the sensory V2. Direct nerve stimulation of the sensory branch V2 at the foramen ovale demonstrating cortical SSEPs recorded from scalp electrodes (montages C4’-Cz’, C4’-Fz, C4’-C3’) accordingly to the international 10–20 EEG system. Average latency: 5.4 ms, amplitude: 12.5 μV.
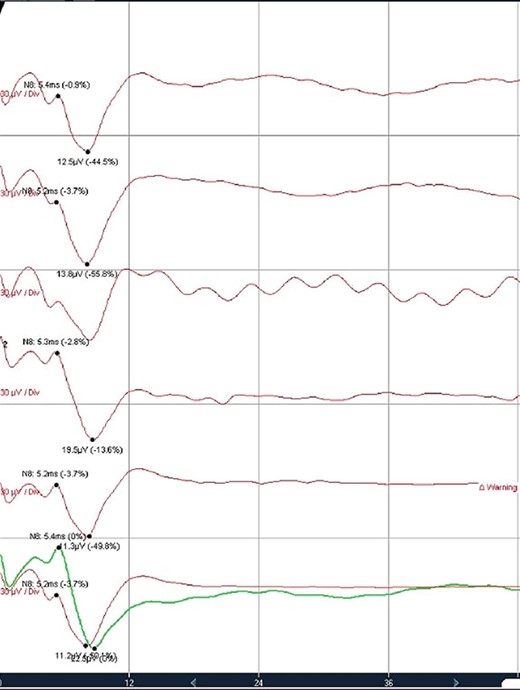
Trigeminal V2 SSEP recordings. Trigeminal V2 SSEPs were continuously recorded on the scalp using the montage C4’-Fz. Average latency: 5.4 ms, amplitude: 13 μV.
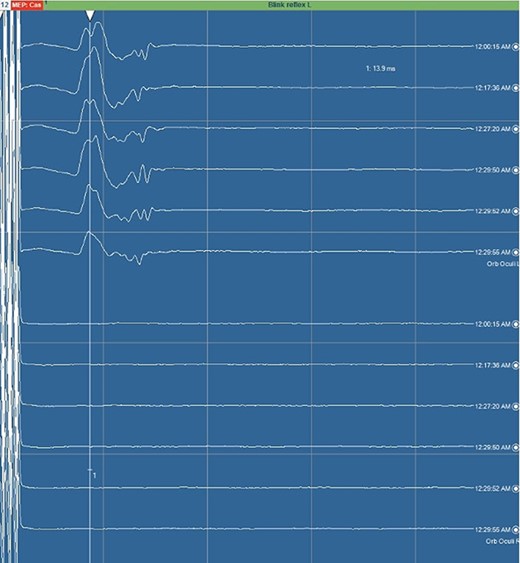
Blink reflex. Blink reflex from left side stimulation of the supraorbital branch of the trigeminal nerve was observed with EMG activity from left orbicularis oculi muscle. Both early response (R1) and late reflex activity (R2) were recorded throughout the surgical procedure.
Neuromonitoring parameters are described in the Supplemental file.
HISTOPATHOLOGY
Histology showed spindle-shaped cells with large nuclei and small amount of cytoplasm, haphazardly arranged around capillaries, along with staghorn vessels consistent with HPC. Ki67 was found to be ~6–7% and the tumor was graded as Grade II (WHO). Immunohistochemistry showed negative EMA and positive CD34.
FOLLOW UP
The 3-month follow-up MRI scan did not reveal any residual, and a whole body Positron Emission Tomography (PET) scan confirmed that there were no extracranial metastases. The patient continues to be followed up for recurrent or metastatic disease.
DISCUSSION
HPCs are rare, comprising less than 1% of all intracranial tumors. Distinguishing them from meningiomas is critical, as these tumors have a worse prognosis, and their rates of recurrence and incidence of extracranial disease dictate a different surveillance and treatment strategy, including the frequent use of adjuvant radiotherapy. Male predominance [5, 6] and younger age [7] are key factors in differentiating from meningiomas. Complete resection is the single most important factor in reducing the incidence of recurrence and increasing survival [5, 6, 8, 9]. However, this is often challenging due to excessive bleeding and adherence to adjacent structures and radiotherapy is often considered post-operatively to prevent recurrence [10]. Thus, the most recent WHO classification of tumors (2021) has assigned these lesions to the entity ‘solitary fibrous tumor of the dura’ [11].
In this report, we present a case of V3 HPC. Our literature search revealed only two previous reports of such tumors. Tan et al. [5] present the case of a 41-year-old male who presented with diplopia and facial paraesthesia and was found to have a right sided lesion measuring 4 cm maximum diameter eroding through Meckel’s cave and petrous apex. The second case report [4] is of a 33-year-old female who presented with a seizure and a preceding 3-week history of left lower limb weakness. The underlying lesion was 5 cm across on the imaging.
Surgical considerations for HPCs of trigeminal nerve are similar to those for trigeminal schwannomas [12]. The initial approach is through a pterional craniotomy, with extradural drilling of the sphenoid wing. Unroofing of foramina ovale and rotundum allows the whole of the trigeminal complex to be mobilized. The inter-dural approach that we used initially is well described and gives good access to the area of the Meckel’s cave [13]. However, dural opening and intra-dural debulking was necessary in our case due to the significant intra-dural component.
Neuromonitoring for trigeminal nerve commonly involves monitoring MEPs from the masseter and temporalis muscles [14]. Although monitoring orbicularis oculi muscle is mainly used to check facial nerve integrity, we used the blink reflex to monitor the function of the supra-orbital branch of trigeminal nerve, acting as the afferent arm of this reflex. We used scalp SSEPs to monitor the sensory function of trigeminal nerve with reproducible results as described in literature [15, 16].
CONCLUSIONS
We describe a rare case of HPC of trigeminal nerve. A combined extradural–intradural approach was effective in debulking this tumor, and intraoperative neuromonitoring of trigeminal nerve was a helpful adjunct in safe tumor resection.
PATIENT CONSENT
The patient has consented to the submission of the case report for submission to the journal. Case Reports is written in accordance with COPE guidelines.
CONFERENCE PRESENTATION
A part of the paper was presented as a poster in British Neuro-Oncology Society (BNOS) Annual Meeting, July 2019.
CONFLICT OF INTEREST STATEMENT
None.
FUNDING
None.



