-
PDF
- Split View
-
Views
-
Cite
Cite
Victoria Cook, Animesh A Singla, David Herlihy, Walid Mohabbat, Charles Fisher, Vikram Puttaswamy, Successful endovascular exclusion of Mycobacterium bovis mycotic aneurysm following intravesical bacille Calmette–Guerin therapy, Journal of Surgical Case Reports, Volume 2022, Issue 10, October 2022, rjac426, https://doi.org/10.1093/jscr/rjac426
Close - Share Icon Share
Abstract
Bacille Calmette–Guerin (BCG) is a live-attenuated strain of Mycobacterium bovis. It is routinely used in the treatment of early-stage transitional cell carcinoma. The development of mycotic aneurysm in the context of prior intra-vesical BCG treatment has not been reported. This case demonstrates a rare but potentially catastrophic vascular complication of BCG. A high index of suspicion is required for any patient presenting with new aneurysmal disease in the context of previous BCG therapy. The value of endovascular surgery as a bridge to definitive surgical repair and diagnostic considerations is discussed.
INTRODUCTION
Bacille Calmette–Guerin (BCG) is a routine treatment for early-stage transitional cell carcinoma. The complications relating to BCG therapy from systemic spread from intra-vesical infiltration are exceedingly rare but previously documented. The presentation can be delayed by years from the time of treatment, and as such, an accurate history is pertinent to appropriate diagnosis. Surgical and endovascular treatment options exist. We report a rare case of BCG treatment with subsequent development of mycotic aneurysm affecting the infrarenal aorta. The pathophysiology and treatment considerations are presented.
CASE REPORT
A 73-year-old male presented with a 1-week history of back pain on a background of hypertension, atrial fibrillation and bladder cancer. Computed tomography (CT) angiography demonstrated a 47 × 46 × 47 mm saccular aortic pseudoaneurysm of the infrarenal aorta with a contained rupture (Fig. 1). The patient remained hemodynamically stable with mildly elevated inflammatory markers (C-Reactive Protein (CRP), 85 mg/l, white cell count (WCC) 6.5 × 109/l). The abdominal aorta 5 months previously was normal on CT when a course of intra-vesical BCG for transitional cell carcinoma of the bladder had been ceased due to the development of fevers and syncope. Thus, Mycobacterium bovis involvement of the false aneurysm was suspected. Emergent endovascular repair was performed with a covered stent graft system successfully excluding the ruptured aneurysm sac on post-operative CT angiogram. A CT-guided fine needle aspirate of the aneurysmal sac was later conducted (Fig. 2). PCR of the aspirate returned a low-positive result confirming the diagnosis with M. bovis subsequently isolated on culture. Empirical antibiotics were ceased and he was commenced on a planned 18-month antituberculosis regimen of ethambutol, isoniazid and rifampicin guided by gene deletion analysis (GeneXpert MTB/RIF Ultra assay). Fluorodeoxyglucose-positron emission tomography (FDG-PET) scan was used to monitor the level of activity of the infection (Fig. 3). The patient was discharged home on Day 10 of admission. Aneurysm sac size was decreased at 3 and 6 months on CT angiogram and FDG-PET but there was ongoing FDG avidity around the aneurysm sac; there were no other sites of involvement. The patient remains otherwise well and has returned to his premorbid activities.
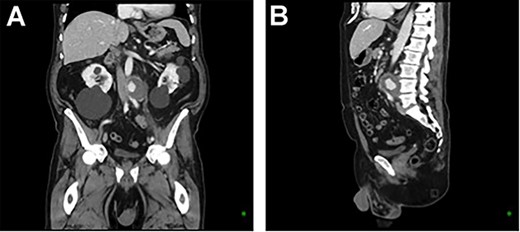
CT aortic angiography demonstrating a 47 × 46 × 47 mm infrarenal aortic pseudoaneurysm with an area of contrast filling and associated mass displacement of the aorta and inferior vena cava. (A) Coronal and (B) sagittal.
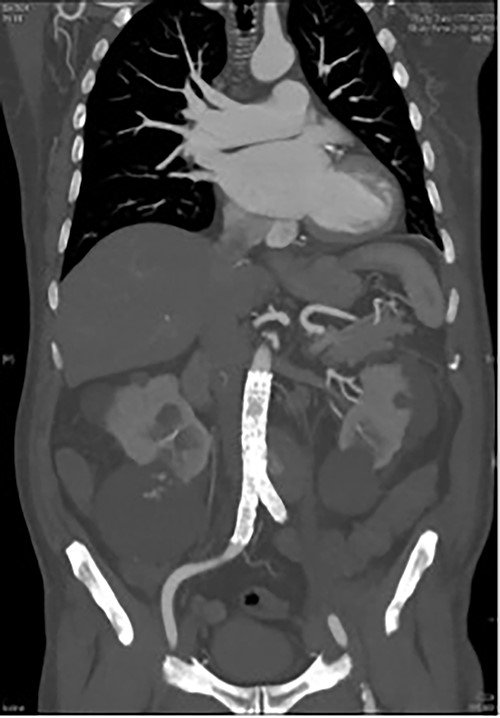
CT angiography post endovascular repair demonstrates an aorto-bi-iliac stent graft extending from the infrarenal aorta to the common iliac arteries with a small area of persistent filling of the pseudoaneurysm that subsequently resolved.
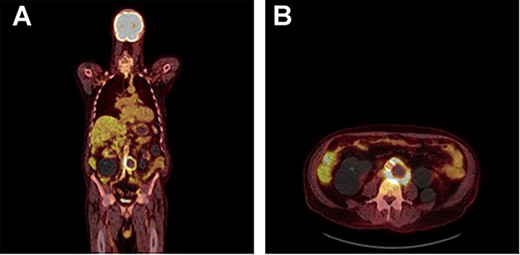
FDG PET/CT study demonstrating a rind of intense FDG uptake (SUV max 13.4) along the circumference of the lateral lower aortic aneurysm abutting the prosthesis however demonstrating no uptake tracking along the prosthesis. No other areas of FDG activity were demonstrated. (A) Coronal and (B) axial.
DISCUSSION
BCG is a live-attenuated strain of M. bovis. Developed as a tuberculosis vaccination, it is now also used as first-line therapy in the treatment of early-stage transitional carcinoma of the bladder via intravesical instillation. This case demonstrates a rare but potentially catastrophic vascular complication of BCG.
A high index of suspicion is required for any patient presenting with new aneurysmal disease in the context of previous BCG therapy. Patients may present up to 5 years post intravesical therapy [1] with weight loss, night sweats or fever but systemic symptoms may also occur at the time of BCG instillation [1]. Infection is through retroperitoneal lymphatics, and hence vascular infections are predominantly in the abdominal aorta and lower limb vasculature in otherwise previously normal vessels rather than seeding of existing atherosclerotic or aneurysmal disease although patients with existing endoprostheses may still be at risk of developing mycotic aneurysms. Subsequent immunosuppression also increases the risk of delayed BCG-related complications including aneurysm formation and should be considered if BCG therapy is planned.
Open surgical treatment comprises excision and ligation of the infected artery, debridement of the infected vasculature bed and extra-anatomical bypass [2] although in aortic cases in-line repair with dacron graft has been performed. There are three reported cases of BCG-associated mycotic aortic aneurysm successfully managed with endovascular repair and concurrent antitubercular therapy [3–6]. Mycobacterium bovis is less virulent than the organisms typically associated with mycotic aneurysms (Staphylococcus spp. and Salmonella spp.) altering the risk graft infection post implantation [3]. Patients treated for aneurysmal disease with unrecognized M. bovis involvement show disease progression with further aneurysmal development, recurrent graft and soft tissue infection, and progression to explant or death regardless of open or endovascular approach [2]. Precise microbiological diagnosis and subsequent appropriate antitubercular therapy may be more critical than the type of vascular procedure.
It remains unclear whether definitive later management with explant and subsequent open repair is necessary in patients who are well with endovascular grafts in situ and are tolerating antitubercular therapy. In our case, significant consideration was given to stent positioning to ensure stent positing would not preclude future open repair in case of progression of disease despite antitubercular therapy (conserving a proximal and distal clamp zone).
A difficulty of endovascular repair is the lack of opportunity to collect intraoperative specimens but microbiological confirmation of BCG should still be pursued [4] as in our case when successful identification of BCG was achieved via CT-guided biopsy of the excluded aneurysmal sac.
CONCLUSION
This case highlights the need for awareness of a rare vascular complication of intravesical BCG for bladder cancer. A history of intravesical BCG instillation should be sought in patients with mycotic aneurysmal disease. Endovascular repair with appropriate antimicrobial therapy was used for emergent management in our patient, accompanied by close monitoring and extended antitubercular therapy.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



