-
PDF
- Split View
-
Views
-
Cite
Cite
Taihei Kajiyama, Masahiro Komori, Mitsuko Iguchi, Junko Nakashima, Asuka Nagao, Masamitsu Hyodo, Laryngeal malignant peripheral nerve sheath tumor mixed with high- and low-grade malignancies, Journal of Surgical Case Reports, Volume 2021, Issue 8, August 2021, rjab373, https://doi.org/10.1093/jscr/rjab373
Close - Share Icon Share
Abstract
Malignant peripheral nerve sheath tumors (MPNSTs), as defined by immunohistochemical evaluation, are identified along a spectrum ranging from atypical neurofibroma to high-grade MPNST because these tumors are similar in terms of cell shape and tissue components on hematoxylin–eosin (HE) staining. The patient was a 57-year-old male referred to our hospital, with a recurrent red tumor at the anterior commissure of the larynx and submucosal swelling of the right vocal fold. A surgical specimen from a right horizontal partial laryngectomy was evaluated immunohistochemically. A high-grade MPNST lesion was included in the submucosal white tumor, whereas a low-grade MPNST lesion was encountered around the high-grade MPNST lesion. This tumor may involve different malignancies even when it is small. Although intra-tumor heterogeneity in cancers has been reported recently, careful immunohistochemical examination can be important and beneficial for eradicating the tumor while preserving vocal function.
INTRODUCTION
Malignant peripheral nerve sheath tumors (MPNSTs) can arise in ~50% of patients with neurofibromatosis Type I, sporadically from benign neurofibroma, or rarely following radiation treatment [1]. In 2017, the histological transformation from atypical neurofibroma to MPNST was classified into four stages [2]: atypical neurofibroma, atypical neurofibromatous neoplasm with uncertain biologic potential (ANNUBP), low-grade MPNST and high-grade MPNST. Given that these tumors are similar in terms of cell shape and tissue components on hematoxylin–eosin (HE) staining, the classifications are identified by immunohistochemical evaluation [2]. In the clinical setting, surgical resection with wide negative margins is necessary to eradicate high-grade MPNSTs [3], whereas the others typically require less aggressive surgery [4]. Additionally, MPNSTs are minimally responsive to chemotherapy [3]. The role of radiotherapy remains limited: although adjuvant radiotherapy is recommended to improve local control, there is no effect on overall survival [5].
| . | HC . | mitoses . | MIB1 . | p53 . | S100 . |
|---|---|---|---|---|---|
| Red tumor and recurrence | |||||
| Previous surgical specimen | (+) | 20% | (+) | (±) | |
| Our surgical specimen by laryngeal microsurgery | (+) | 21/10 HPF | 20% | (+) | (±) |
| Submucosal white tumor | |||||
| Biopsy | |||||
| (1) anterior commissure of larynx | (+) | (+) | (+) | ||
| (2) midmembranous v. c. | (+) | (+) | (+) | ||
| (3) around vocal process | (±) | (±) | (±) | ||
| (4) ventral side of the posterior v. c. | (±) | (+) | (+) | ||
| (5) dorsal side of the posterior v. c. | (–) | (–) | (–) | ||
| Surgical specimen by the right horizontal partial laryngectomy | (±)~(+) | 9.1~13.7% | (±)~(+) | ||
| . | HC . | mitoses . | MIB1 . | p53 . | S100 . |
|---|---|---|---|---|---|
| Red tumor and recurrence | |||||
| Previous surgical specimen | (+) | 20% | (+) | (±) | |
| Our surgical specimen by laryngeal microsurgery | (+) | 21/10 HPF | 20% | (+) | (±) |
| Submucosal white tumor | |||||
| Biopsy | |||||
| (1) anterior commissure of larynx | (+) | (+) | (+) | ||
| (2) midmembranous v. c. | (+) | (+) | (+) | ||
| (3) around vocal process | (±) | (±) | (±) | ||
| (4) ventral side of the posterior v. c. | (±) | (+) | (+) | ||
| (5) dorsal side of the posterior v. c. | (–) | (–) | (–) | ||
| Surgical specimen by the right horizontal partial laryngectomy | (±)~(+) | 9.1~13.7% | (±)~(+) | ||
(–) indicates negative; (±): sporadically positive; (+): positive/over expression; HC: hyperchromatic; HPF: high-power fields; v. c.: vocal cord
| . | HC . | mitoses . | MIB1 . | p53 . | S100 . |
|---|---|---|---|---|---|
| Red tumor and recurrence | |||||
| Previous surgical specimen | (+) | 20% | (+) | (±) | |
| Our surgical specimen by laryngeal microsurgery | (+) | 21/10 HPF | 20% | (+) | (±) |
| Submucosal white tumor | |||||
| Biopsy | |||||
| (1) anterior commissure of larynx | (+) | (+) | (+) | ||
| (2) midmembranous v. c. | (+) | (+) | (+) | ||
| (3) around vocal process | (±) | (±) | (±) | ||
| (4) ventral side of the posterior v. c. | (±) | (+) | (+) | ||
| (5) dorsal side of the posterior v. c. | (–) | (–) | (–) | ||
| Surgical specimen by the right horizontal partial laryngectomy | (±)~(+) | 9.1~13.7% | (±)~(+) | ||
| . | HC . | mitoses . | MIB1 . | p53 . | S100 . |
|---|---|---|---|---|---|
| Red tumor and recurrence | |||||
| Previous surgical specimen | (+) | 20% | (+) | (±) | |
| Our surgical specimen by laryngeal microsurgery | (+) | 21/10 HPF | 20% | (+) | (±) |
| Submucosal white tumor | |||||
| Biopsy | |||||
| (1) anterior commissure of larynx | (+) | (+) | (+) | ||
| (2) midmembranous v. c. | (+) | (+) | (+) | ||
| (3) around vocal process | (±) | (±) | (±) | ||
| (4) ventral side of the posterior v. c. | (±) | (+) | (+) | ||
| (5) dorsal side of the posterior v. c. | (–) | (–) | (–) | ||
| Surgical specimen by the right horizontal partial laryngectomy | (±)~(+) | 9.1~13.7% | (±)~(+) | ||
(–) indicates negative; (±): sporadically positive; (+): positive/over expression; HC: hyperchromatic; HPF: high-power fields; v. c.: vocal cord
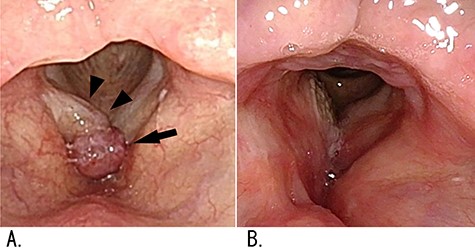
Findings of laryngeal flexible-endoscopy examination; (A) preoperative findings: a red tumor on the anterior commissure of the larynx (arrow) and a diffuse swelling of the right vocal fold (arrowheads) were identified; (B) no recurrence was found 3.5 years after surgery.
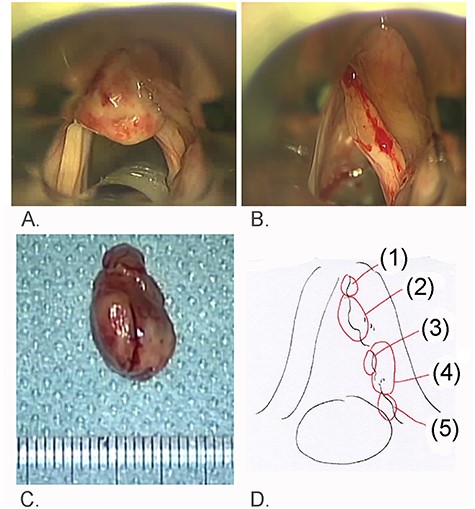
Findings of laryngeal microsurgery; (A) a recurrent red tumor was encountered at the anterior commissure of the larynx; (B) swelling of the right vocal fold was identified after removal of the red tumor; (C) the red tumor measured 15 × 9 mm and was soft; (D) numbers indicate biopsied areas of the right vocal fold.
MPNST in the head and neck is rare, although it is more aggressive than at other sites [6]. Its recurrence rate is high: there is local recurrence in up to 50% of cases and metastasis to the lung and bone in up to 33%. Lymphatic metastases are infrequent [1]. The 5-year overall survival depends on the completeness of surgical extraction as well as on the tumor size and degree of histological differentiation [1, 6].
Here, we report a case of laryngeal MPNST with low- and high-grade malignancies. In the larynx, benign schwannoma and benign neurofibroma are rare: only ~50 and 30 cases, respectively, have been reported [7, 8]. Rarer still are reports of MPNSTs involving the larynx [9–12].
CASE REPORT
Written informed consent was obtained from the patient for this case report.
A 57-year-old male presented at another hospital with hoarseness that had gradually worsened over a 4-year period. No pain was present. A laryngeal flexible-endoscopy examination revealed a 5-mm diameter red tumor at the anterior commissure of the larynx and a swelling of the right vocal fold. Resection of the red tumor and biopsy of a white submucosal tumor on the right vocal fold were performed by means of laryngeal microsurgery. Histopathological examination indicated that the possibility of malignancy could not be excluded. The red tumor then recurred at the same site 4 months after the resection, and the patient was referred to our hospital. Neither neurological signs nor skin signs such as cafe au lait spots were found. The patient had never received radiation therapy and had no co-morbidities. He had a history of smoking 20–40 cigarettes a day and drinking 500 ml of beer daily.
![Histopathological and immunohistochemical findings of the surgical specimen from right horizontal partial laryngectomy; (A) photograph of the surgical specimen; upper side is cranial one, left side is the anterior of the tumor and right side is the posterior of the tumor; line indicates location of sectioning; (B) HE staining of the tumor; upper side is cranial one, left side is the inside of the tumor and right side is the outside of the tumor; dotted line indicates the area of high-grade malignancy [1]: part of the low-grade MPNST; [2]: part of the high-grade MPNST; (C–F) low-grade MPNST [area (1) in B]; (C) there were a number of spindle cells, but the size of the nuclei varied and only some were hyperchromatic on HE staining; (D) the MIB-1 labeling index was 9.1%; (E) some of the spindle cells were positive on S100 staining; (F) area of dotted line box in (E) points to tumor cells infiltrating the internal laryngeal muscle; (F–H) high-grade MPNST [area (2) in B]; (F) there were a number of spindle cells with large nuclei that were hyperchromatic on HE staining; (G) the MIB-1 labeling index was 13.7%; (H) few spindle cells with S100 protein expression were found; (C, F) HE stain; (D, G) MIB-1 stain; (E, H) S100 stain; scale bars: 100 μm; boxes at bottom right are magnifications of the areas delineated by the line rectangles.](https://oupdevcdn.silverchair-staging.com/oup/backfile/Content_public/Journal/jscr/2021/8/10.1093_jscr_rjab373/1/m_rjab373f3.jpeg?Expires=1772429772&Signature=c8jUNUKtpcCzzU3i2wGM8zhSJV-BH7XFlWGs6A4wKqiEiciCGFeDqdA4Y4m9aMemYLolX-5zkZXnTExL7edqO1oypMlhfc4fNyT5g2QoSjvo3uywkwEEbMiSOZE6Jn2m6NdeLscVYBxSBW~ut5nkryNzohCNwrTBE-hsaL~l3W4iGAUK4umnvJyRc~BAee-aLTt0I5ZyPNFvrw7qiOcf1PbvYPimsxtimw1xtLOp6xgTGlo-qafcG3fLOAL-r~1pEM~xD5c6~m7NcPy6EA12GCrbKS0bzyPbAeYDi0ARR4exLZQM43M3aD-LRBuLGNJwpMXnQJfLct0yiamz3vBbEg__&Key-Pair-Id=APKAIYYTVHKX7JZB5EAA)
Histopathological and immunohistochemical findings of the surgical specimen from right horizontal partial laryngectomy; (A) photograph of the surgical specimen; upper side is cranial one, left side is the anterior of the tumor and right side is the posterior of the tumor; line indicates location of sectioning; (B) HE staining of the tumor; upper side is cranial one, left side is the inside of the tumor and right side is the outside of the tumor; dotted line indicates the area of high-grade malignancy [1]: part of the low-grade MPNST; [2]: part of the high-grade MPNST; (C–F) low-grade MPNST [area (1) in B]; (C) there were a number of spindle cells, but the size of the nuclei varied and only some were hyperchromatic on HE staining; (D) the MIB-1 labeling index was 9.1%; (E) some of the spindle cells were positive on S100 staining; (F) area of dotted line box in (E) points to tumor cells infiltrating the internal laryngeal muscle; (F–H) high-grade MPNST [area (2) in B]; (F) there were a number of spindle cells with large nuclei that were hyperchromatic on HE staining; (G) the MIB-1 labeling index was 13.7%; (H) few spindle cells with S100 protein expression were found; (C, F) HE stain; (D, G) MIB-1 stain; (E, H) S100 stain; scale bars: 100 μm; boxes at bottom right are magnifications of the areas delineated by the line rectangles.
At the initial visit to our hospital, a laryngeal flexible-endoscopy examination showed a red tumor on the anterior commissure of the larynx and a diffuse swelling of the right vocal fold (Fig. 1A). Immunohistochemical examination of the previous surgical specimen indicated the possibility of MPNST because hyperchromatic spindle cells in which MIB-1 labeling index was 20%, P53 protein was overexpression and S100 protein was sporadically positive were identified (Table). In addition, Sox10, CD34 and p16 protein expression was negative. Removal of the recurrent red tumor and several biopsies at different sites in the white submucosal tumor of the right vocal fold were performed again (Fig. 2). The removed red tumor measured 15 × 9 mm. The recurrent red tumor and associated swelling on the anterior commissure of larynx, as well as the midmembranous vocal fold lesions (biopsy 2 in Fig. 2D), were high-grade MPNSTs immunohistochemically; the lesion on the ventral side of the right posterior vocal cord (biopsy 4 in Fig. 2D) was less malignant than lesion of biopsies 1 and 2. The lesion on the dorsal side of the same vocal fold (biopsy 5 in Fig. 2D) did not involve any tissue of the tumor.
Thereafter, a right horizontal partial laryngectomy was performed. The tumor was 9 mm in diameter. A high-grade MPNST lesion was included in the submucosal white tumor immunohistochemically, whereas a low-grade MPNST lesion was encountered around the high-grade MPNST lesion (Fig. 3B). In the low-grade MPNST lesion, there were a number of spindle cells, but the size of the nuclei varied and only some were hyperchromatic. The spindle cells had an MIB-1 labeling index of 9.1% and sporadic S100 protein expression (Fig. 3C–E). A safe margin of resection was confirmed, although some tumor cells had infiltrated the internal laryngeal muscle (Fig. 3E and F). By contrast, in the high-grade MPNST lesion, a number of spindle cells with large and hyperchromatic nuclei were identified. These spindle cells had a mind-bomb homolog 1 (MIB-1) labeling index of 13.7% and focally S100 protein expression (Fig. 3G–I).
To date, neither local recurrences (Fig. 1B) nor metastases have been found 3.5 years after surgery. The patient’s vocal function has been well preserved.
DISCUSSION
Careful immunohistochemical examination can be important to eradicating laryngeal MPNSTs. In our patient, the surgical margin was considered sufficient for low-grade MPNST. However, from our evaluation using surgical specimen by the right horizontal partial laryngectomy, we considered that the margin might not have been enough if the low-grade MPNST lesion was not around the high-grade MPNST lesion. Although intra-tumor heterogeneity in cancers has been reported recently [13], immunohistochemical evaluations at several different sites of the tumor, as well as the immediate additional excision, likely allowed us to maintain our patient’s vocal function and improve his long-term prognosis.
The features of laryngeal MPNST have been discussed on the previous reports [9–12]. This type of tumor was identified submucosally in a previous study [11] and in our patient. Ipsilateral vocal fold palsy was present in one case [11]. No patients with pain have been reported. One underwent partial laryngectomy but died from distant metastases 6 months later [9]. Another patient underwent total laryngectomy and adjuvant radiotherapy and lived for 5 years [12]. Particularly in the larynx, small tumors can now be identified by using modern imaging and endoscopic modalities. Our results indicate that even these small laryngeal MPNSTs can involve various degrees of malignancy.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
We received no financial or material support for the study.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.



